
VOLUME 105 | ISSUE 4S | APRIL 2024
www.kidney-international.org
KDIGO 2024 Clinical Practice Guideline for the
Evaluation and Management of Chronic Kidney Disease
SUPPLEMENT TO

KDIGO 2024 Clinical Practice Guideline for the Evaluation and
Management of Chronic Kidney Disease
S118 Tables, figures, and supplementary material
S124 KDIGO Executive Committee
S125 Reference keys
S126 CKD nomenclature
S127 Conversion factors
S128 Abbreviations and acronyms
S129 Notice
S130 Foreword
S131 Work Group membership
S133 Abstract
S134 Patient foreword
S135 Introduction, qualifying statements, and key concepts
S141 Special considerations
S144 Summary of relative and absolute risks relevant to CKD from meta-analysis of
large multinational population studies in the CKD Prognosis Consortium
(CKD-PC)
S149 Summary of recommendation statements and practice points
S169 Chapter 1: Evaluation of CKD
S196 Chapter 2: Risk assessment in people with CKD
S205 Chapter 3: Delaying CKD progression and managing its complications
S246 Chapter 4: Medication management and drug stewardship in CKD
S255 Chapter 5: Optimal models of care
S270 Chapter 6: Research recommendations
S274 Methods for guideline development
S283 Biographic and disclosure information
S294 Acknowledgments
S295 References
This article is published as part of a supplement sponsored by Kidney Disease: Improving Global Outcomes (KDIGO). The
opinions or views expressed in this supplement are those of the authors and do not necessarily reflect the opinions or rec-
ommendations of the International Society of Nephrology or Elsevier. Dosages, indications, and methods of use for products
that are referred to in the supplement by the authors may reflect their clinical experience or may be derived from the pro-
fessional literature or other clinical sources. Because of the differences between in vitro and in vivo systems and between
laboratory animal models and clinical data in humans, in vitro and animal data do not necessarily correlate with clinical results.
contents www.kidney-international.org
VOL 105 | ISSUE 4S | APRIL 2024
S118 Kidney International (2024) 105 (Suppl 4S), S117–S314

TABLES
S137 Table 1. Criteria for chronic kidney disease
S137 Table 2. GFR categories in CKD
S137 Table 3. Albuminuria categories in chronic kidney disease (CKD)
S169 Table 4. Use of GFR and albuminuria
S171 Table 5. Risk factors for CKD
S174 Table 6. Guidance for selection of additional tests for evaluation of cause
S178 Table 7. Description of initial and supportive tests for evaluation of GFR
S179 Table 8. Indications for use of cystatin C
S180 Table 9. Comparison of estimated GFR and measured GFR
S181 Table 10. Indications for measured GFR
S184 Table 11. Implementation standards to ensure accuracy and reliability of GFR assessments using creatinine and
cystatin C
S184 Table 12. Reported examples of substances that may cause analytical interferences in creatinine assays
S187 Table 13. Criteria for a validated GFR estimating equation
S189 Table 14. Validated GFR estimating equations
S190 Table 15. Criteria for equation comparison for comparison of candidate equations to another (i.e., how to
determine validity)
S191 Table 16. Factors causing biological variation in urine albumin or urine protein
S193 Table 17. Implementation standards to ensure accuracy and reliability of urine samples
S198 Table 18. Impact of albuminuria/proteinuria on CKD progression in pediatrics
S199 Table 19. Externally validated risk equations for predicting kidney failure in the general (CKD G3–G5)
population
S203 Table 20. Externally validated risk equations for predicting a 40% decline in GFR
S209 Table 21. Impact of plant-based foods in people with CKD
S212 Table 22. Age-based sodium intake recommendations
S222 Table 23. Variation of laboratory values in a large population database by age group, sex, and eGFR; bicarbonate,
mmol/l, mean (SD), n [ 3,990,898
S223 Table 24. Variation of laboratory values in a large population database by age group, sex, and eGFR; potassium,
mmol/l, mean (SD), n [ 4,278,600
S225 Table 25. Factors and mechanisms that impact on potassium measurements
S226 Table 26. Medications associated with increased risk of hyperkalemia
S227 Table 27. A comparison of potassium exchange agents
S227 Table 28. Suggested action in the event of moderate and severe hyperkalemia
S229 Table 29. Variation of laboratory values in a large population database by age group, sex, and eGFR; hemoglobin,
g/dl, mean (SD), n [3,561,622
S233 Table 30. Randomized controlled trials in the treatment of asymptomatic hyperuricemia in people with CKD
S247 Table 31. Key examples of common medications with documented nephrotoxicity and, where available, selected
non-nephrotoxic alternatives
S252 Table 32. Medications that should be considered for temporary discontinuation before elective surgeries and
potential perioperative adverse events associated with their continued use
S253 Table 33. Potential risk factors for contrast-associated acute kidney injury
S256 Table 34. Benefits and consequences of early versus late referral
S257 Table 35. Factors associated with late referral for kidney replacement therapy planning
S257 Table 36. Outcomes examined in a systematic review by Smart et al.
S258 Table 37. Recommended patient-reported outcome measurement tools for use in people with CKD
www.kidney-international.org contents
Kidney International (2024) 105 (Suppl 4S), S117–S314 S119

S259 Table 38. Management strategies for common symptoms in CKD
S261 Table 39. List of validated assessment tools for malnutrition
S262 Table 40. Key features of existing CKD care models
S266 Table 41. Indications for the initiation of dialysis
S267 Table 42. Studies examining the timing of dialysis in people with CKD
S268 Table 43. People with kidney failure who receive comprehensive conservative care
S275 Table 44. Clinical questions and systematic review topics in PICOS format
S280 Table 45. Classification for certainty of evidence
S280 Table 46. GRADE system for grading the certainty of evidence
S281 Table 47. KDIGO nomenclature and description for grading recommendations
S281 Table 48. Determinants of the strength of recommendation
FIGURES
S136 Figure 1. Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by
creatinine and cystatin C (eGFRcr-cys) and albumin-to-creatinine ratio (ACR) categories an d risks for
10 common complications by age in multivariable-adjusted analyses
S138 Figure 2. Age-standardized chronic kidney disease disability-adju sted life-year (DALY) rates for each location by
sociodemographic index, both sexes combined, 2019
S139 Figure 3. Screening algorithm for diagnosis and staging of chronic kidney disease (CKD) in adults
S141 Figure 4. Special considerations for chronic kidney disease (CKD) care across the lifespan
S145 Figure 5. Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by
creatinine (eGFRcr) and albumin-to-creatinine ratio (ACR) categories and risks for 10 common
complications in multivariable-adjusted analyses
S146 Figure 6. Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by
creatinine and cystatin C (eGFRcr-cys) and albumin-to-creatinine ratio categories and risks for 10
common complications in multivariable-adjusted analyses
S147 Figure 7. Hazard ratios for adverse outcomes using the continuous model of estimated glomerular filtration rate
(eGFR), comparison of the shape of associations between creatinine-based eGFR (eGFRcr) and
creatinine and cystatin C–based eGFR (eGFRcr-cys) in the population with cystatin C (eGFRcr-cys
population)
S173 Figure 8. Evaluation of cause of chronic kidney disease (CKD)
S174 Figure 9. Actionable genes in kidney disease
S175 Figure 10. Proposed organization for implementing genetics in nephrology
S177 Figure 11. Approach to glomerular filtration rate (GFR) evaluation using initial and supportive tests
S182 Figure 12. Sources and magnitude of error around measured glomerular filtration rate (mGFR) and estimated GFR
(eGFR)
S197 Figure 13. Frequency of monitoring glomerular filtration rate (GFR) and albuminuria in people with chronic
kidney disease (CKD)
S199 Figure 14. (a) Predicted risk of kidney failure and (b) ‡40% decline in estimated glomerular filtration rate (eGFR)
by chronic kidney disease (CKD) eGFR (G1–G5) and albumin-to-creatinine ratio (ACR) (A1–A3)
categories in Optum Labs Data Warehouse
S201 Figure 15. Transition from an estimated glomerular filtration rate (eGFR)-based to a risk-based approach to
chronic kidney disease care
S202 Figure 16. Comparison of risk of chronic kidney disease (CKD) progression (5-year probability of estimated
glomerular filtration rate [eGFR] <60 ml/min per 1.73 m
2
) versus kidney failure in adults with CKD G1–
G2 calculated from the risk equation available at https://www.ckdpc.org/risk-models.html
S205 Figure 17. Chronic kidney disease (CKD) treatment and risk modification
S206 Figure 18. Holistic approach to chronic kidney disease (CKD) treatment and risk modification
S208 Figure 19. Protein guideline for adults with chronic kidney disease not treated with dialysis
contents www.kidney-international.org
S120
Kidney International (2024) 105 (Suppl 4S), S117–S314

S209 Figure 20. Average protein content of foods in grams
S214 Figure 21. Algorithm for monitoring of potassium and estimated glomerular filtration rate (eGFR) after the initiation
of renin-angiotensin system inhibitors
S215 Figure 22. Effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) with kidney disease outcomes by diabetes
status
S216 Figure 23. Effects of sodium-glucose cotransporter-2 (SGLT2) inhibition versus placebo on cardiovascular and
mortality outcomes by diabetes status and trial population
S217 Figure 24. Effects of sodium-glucose cotransporter-2 (SGLT2) inhibition versus placebo on kidney failure (chronic
kidney disease [CKD] trials)
S219 Figure 25. Effects of empagliflozin versus placebo on annual rate of change in estimated glomerular filtration rate
(GFR) by key subgroups in the Study of Heart and Kidney Protection With Empagliflozin (EMPA-
KIDNEY)
S220 Figure 26. Serum potassium monitoring during treatment with a nonsteroidal mineralocorticoid receptor
antagonist (MRA) (finerenone)
S221 Figure 27. Effect of finerenone versus placebo on kidney and cardiovascular outcomes in pooled analyses from the
Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-
DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease
(FIGARO-DKD trials)
S222 Figure 28. Association between estimated glomerular filtration rate (eGFR) with serum bicarbonate
concentration in general population and h igh-risk cohorts from the Chronic Kidney Disease
Prognosis Consortium, by level of albuminuria (A1–A3)
S224 Figure 29. Distribution of blood potassium in general population and high-risk cohorts from the Chronic Kidney
Disease Prognosis Consortium, by estimated glomerular filtration rate (eGFR)
S224 Figure 30. Meta-analyzed adjusted prevalence of hyperkalemia (25th and 75th percentile cohort) in general
population and high-risk cohorts from the Chronic Kidney Disease Prognosis Consortium, by diabetes
status
S225 Figure 31. Serum potassium concentration and confounder-adjusted risk of death by presence or absence of
diabetes, heart failure (HF), or chronic kidney disease (CKD)
S228 Figure 32. Actions to manage hyperkalemia (potassium >5.5 mmol/l) in chronic kidney disease
S228 Figure 33. Potassium absorption rates of plant-based, animal-based, and processed foods
S229 Figure 34. Association between estimated glomerular filtration rate (eGFR) and hemoglobin concentration from
general population and high-risk cohorts from the Chronic Kidney Disease Prognosis Consortium, by
diabetes status
S230 Figure 35. Association between estimated glomerular fi ltration rate (eGFR) with serum concentrations of
parathyroid hormone, phosphate, and serum calcium in general population and high-risk cohorts
from the Chronic Kidney Disease Prognosis Consortium, by level of albuminuria (A1–A3)
S233 Figure 36. Risk of all-cause and cardiovascular mortality by estimated glomerular filtration rate (eGFR) and level of
albuminuria from general population cohorts contributing to the Chronic Kidney Disease Prognosis
Consortium
S235 Figure 37. Effect of lowering low-density lipoprotein (LDL) cholesterol per 1.0 mmol/l on risk of major vascular
events by level of estimated glomerular filtration rate (eGFR) at recruitment
S237 Figure 38. Predicted 5-year absolute benefits and harms of allocation to aspirin (A) versus control (C) using a
secondary or primary prevention strategy, by different levels of risk (based on age and sex)
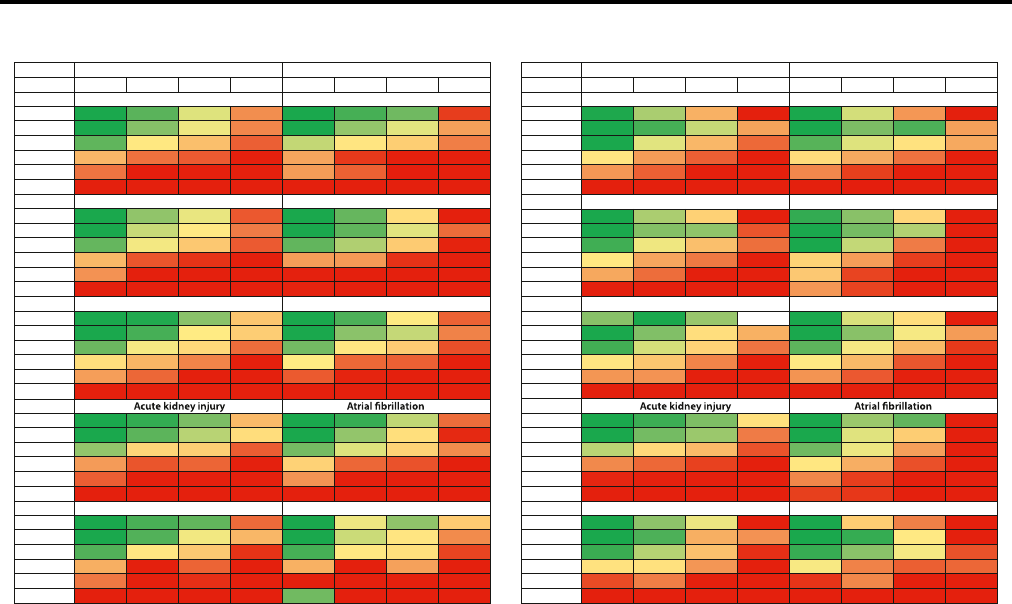
S240 Figure 39. Meta-analyzed adjusted prevalence of atrial fibrillation from cohorts contributing to the Chronic Kidney
Disease Prognosis Consortium, by diabetes status
S241 Figure 40. Strategies for the diagnosis and management of atrial fibrillation
S242 Figure 41. Pooled hazard ratio (HR) comparing non–vitamin K antagonist oral anticoagulants (NOACs) with
warfarin among people with chronic kidney disease in terms of stroke
S243 Figure 42. Pooled hazard ratio (HR) comparing non–vitamin K antagonist oral anticoagulants (NOACs) with
warfarin among people with chronic kidney disease in terms of bleeding
S244 Figure 43. Evidence from (a) randomized controlled trials (RCTs) regarding therapeutic anticoagulation dose by
glomerular filtration rate (GFR) and (b) in areas where RCTs are lacking
S245 Figure 44. Advice on when to discontinue non–vitamin K antagonist oral anticoagulants (NOACs) before
procedures
(low vs. high risk)
www.kidney-international.org contents
Kidney International (2024) 105 (Suppl 4S), S117–S314 S121

S248 Figure 45. Selected herbal remedies and dietary supplements with evidence of potential nephrotoxicity, grouped
by the continent from where the reports first came
S250 Figure 46. Suggested steps in the process of medication review and reconciliation
S251 Figure 47. Essential steps for appropriate sick day rule implementation
S255 Figure 48. Circumstances for referral to specialist kidney care services and goals of the referral
S258 Figure 49. Common symptoms, prevalence, and severity in people with chronic kidney disease
S261 Figure 50. Optimal care model by increasing severity of chronic kidney disease (CKD)
S262 Figure 51. The chronic care model
S262 Figure 52. Specific components of the chronic kidney disease model of care
S263 Figure 53. Strategy for effective patient education programs for people with chronic kidney disease (CKD)
S264 Figure 54. Telehealth technologies for people with chronic kidney disease (CKD)
S265 Figure 55. The process of transition from pediatric to adult care in chronic kidney disease (CKD)
S269 Figure 56. Relationship between supportive care, comprehensive conservative care, and end-of-life care
S279 Figure 57. Search yield and study flow diagram
SUPPLEMENTARY MATERIAL
Supplementary File (PDF)
Appendix A. Search strategies
Table S1. Search strategies for systematic review topics
Appendix B. Concurrence with Institute of Medicine (IOM) standards for guideline development
Table S2. Guideline development checklist – IOM standards for development of trustworthy clinical practice
guidelines
Appendix C. Data supplement - Summary of findings (SoF) tables cited in the guideline text
Chapter 1. Evaluation of CKD
Table S3. Adults and children with or without CKD, estimated GFR (eGFR) based on measurements of cystatin C
(eGFRcys); creatinine (eGFRcr); cystatin C and creatinine (eGFRcr-cys) versus measured GFR (mGFR;
using urinary or plasma clearance of exogenous filtration marker)
Table S4. Adults and children with suspected or diagnosed CKD, native kidney biopsy versus clinical or standard
diagnosis or prognosis for studies evaluating diagnostic or prognostic benefit; no comparator for
studies evaluating safety
Table S5. Adults and children, machine-read quantitative or semiquantitative protein or albumin urine dipstick
tests versus laboratory-based methods for measuring urinary protein or albumin (e.g., 24-hour urinary
sample, spot urine protein-to-creatinine ratio [PCR], or albumin-to-creatinine ratio [ACR])
Chapter 2. Risk assessment in people with CKD
Table S6. Adults, children, and young people with CKD G1–G5, C-statistics of kidney failure risk equations for
predicting progression (e.g., Tangri equation [KFRE])
Table S7. Adults, children, and young people with CKD G1–G5, Brier scores of kidney failure risk equations for
predicting progression (e.g., Tangri equation [KFRE])
Table S8. Adults, children, and young people with CKD G1–G5, R
2
statistics of kidney failure risk equations for
predicting progression (e.g., Tangri equation [KFRE])
Table S9. Adults, children, and young people with CKD G1–G5, sensitivity and specificity to start kidney
replacement therapy (KRT) for kidney failure risk equations for predicting progression (e.g., Tangri
equation [KFRE])
Chapter 3. Delaying CKD progression and managing its complications
Table S10. Adults and children with CKD, sodium-glucose cotransporter-2 inhibitors (SGLT2i) versus placebo or
usual care; active comparator (e.g., another glucose-lowering agent)
Table S11. Adults and children with CKD and symptomatic hyperuricemia, uric acid–lowering therapy (ULT;
allopurinol, benzbromarone, febuxostat, oxipurinol, pegloticase, probenecid, topiroxostat,
rasburicase, sulfinpyrazone, lesinurad) versus active comparator, placebo, or usual care
Table S12. Adults and children with CKD and asymptomatic hyperuricemia, uric acid–lowering therapy (ULT;
allopurinol, benzbromarone, febuxostat, oxipurinol, pegloticase, probenecid, topiroxostat,
rasburicase, sulfinpyrazone, lesinurad) versus active comparator, placebo, or usual care
Table S13. Adults and children with CKD and ischemic heart disease, angiography or coronary revascularization
versus medical treatment
contents www.kidney-international.org
S122
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table S14. Adults and children with CKD and atrial fibrillation, non–vitamin K antagonist oral anticoagulant
(NOAC) with warfarin or NOAC alone versus medical treatment—stroke outcomes
Table S15. Adults and children with CKD and atrial fibrillation, non–vitamin K antagonist oral anticoagulant
(NOAC) with warfarin or NOAC alone versus medical treatment—bleeding outcomes
Appendix D – Data supplement - Summary of findings (SoF) tables not cited in the guideline text
Chapter 3. Delaying CKD progression and managing its complications
Table S16. Adults and children with CKD but not type 2 diabetes, steroidal mineralocorticoid receptor agonists
(MRAs; canrenone, eplerenone, spironolactone) or non-steroidal MRAs (finerenone, esaxerenone) versus
active comparator, placebo, or usual care
Table S17. Adults and children with CKD at risk for cardiovascular disease (CVD), aspirin versus placebo
Appendix E – PRISMA diagrams
Chapter 1. Evaluation of CKD
Figure S1. PRISMA diagram for the clinical question “What is the diagnostic and prognostic benefitandsafetyof
kidney biopsy among people with CKD?”
Figure S2. PRISMA diagram for the clinical question “What is the diagnostic accuracy of eGFR based on
measurements of cystatin C, creatinine, or their combination compared to mGFR among people with
and without CKD?”
Figure S3. PRISMA diagram for the clinical question “In children and young adults with suspected or diagnosed
CKD, what is the accuracy of ACR and PCR compared to 24-hour excretion of albumin or protein?”
Figure S4. PRISMA diagram for the clinical question “What is the diagnostic accuracy and reproducibility of POC
blood creatinine compared to laboratory-based tests among people with suspected or diagnosed
CKD?”
Figure S5. PRISMA diagram for the clinical question “What is the diagnostic accuracy of quantitative and
semiquantitative protein or albumin urine dipstick tests compared to laboratory-based tests among
people with suspected or diagnosed CKD?”
Chapter 3. Delaying CKD progression and managing its complications
Figure S6. PRISMA diagram for the clinical question “What is the effect of SGLT2i compared with placebo, usual
care, or an active comparator among people with CKD in terms of mortality, progression of CKD,
complications of CKD, and adverse events?”
Figure S7. PRISMA diagram for the clinical question “What is the effect of MRAs compared with placebo, usual
care, or an active comparator among people with CKD but not type 2 diabetes in terms of mortality,
progression of CKD, complications of CKD, and adverse events?”
Figure S8. PRISMA diagram for the clinical question “What is the effect of glucagon-like peptide-1 (GLP-1)
receptor agonists compared with placebo, usual care, or an active comparator among people with
CKD but not type 2 diabetes in terms of mortality, progression of CKD, complications of CKD, and
adverse events?”
Figure S9. PRISMA diagram for the clinical question “What is the effect of uric acid–lowering therapy compared
with placebo, usual care, or an active comparator among people with CKD and hyperuricemia in terms
of mortality, progression of CKD, complications of CKD, and adverse events?”
Figure S10. PRISMA diagram for the clinical question “What is the effect of aspirin compared to placebo in terms of
the primary prevention of cardiovascular disease (CVD) and safety among people with CKD?”
Figure S11. PRISMA diagram for the clinical question “What are the effects of angiography or coronary
revascularization compared to medical treatment among people with CKD and ischemic heart disease
in terms of mortality, CVD events, kidney failure, and acute kidney injury (AKI)?”
Figure S12. PRISMA diagram for the clinical question
“What
are the effects of NOACs with or without warfarin
compared to placebo or warfarin alone among people with CKD and atrial fibrillation in terms of
stroke and bleeding risks?”
www.kidney-international.org contents
Kidney International (2024) 105 (Suppl 4S), S117–S314 S123

KDIGO EXECUTIVE COMMITTEE
Garabed Eknoyan, MD
Norbert Lameire, MD, PhD
Founding KDIGO Co-Chairs
Wolfgang C. Winkelmayer, MD, MPH, ScD
Immediate Past Co-Chair
Michel Jadoul, MD
KDIGO Co-Chair
Morgan E. Grams, MD, PhD, MHS
KDIGO Co-Chair
Gloria E. Ashuntantang, MD
Sunita Bavanandan, MBBS
Irene de Lourdes Noronha, MD, PhD
Michelle R. Denburg, MD, MSCE
Joachim H. Ix, MD, MAS
Vivekanand Jha, MD, DM, FRCP, FAMS
Holly Kramer, MD, MPH
Adrian Liew, MD, MBBS, MRCP, FAMS, FASN, FRCP, MClinEpid
Reem A. Mustafa, MD, PhD, MPH
Michelle M. O’Shaughnessy, MB, BCh, BAO, MS, MD
Patrick Rossignol, MD, PhD
Paul E. Stevens, MB, FRCP
Rita S. Suri, MD, MSc
Irma Tchokhonelidze, MD
Marc G. Vervloet, MD, PhD, FERA
Wolfgang C. Winkelmayer, MD, MPH, ScD
Motoko Yanagita, MD, PhD
KDIGO Staff
John Davis, Chief Executive Officer
Danielle Green, Executive Director
Melissa Thompson, Chief Operating Officer
Michael Cheung, Chief ScientificOfficer
Amy Earley, Guideline Development Director
Jennifer King, Director of Medical Writing
Tanya Green, Events Director
Coral Cyzewski, Events Coordinator
Kathleen Conn, Director of Communications
KDIGO executive committee www.kidney-international.org
S124
Kidney International (2024) 105 (Suppl 4S), S117–S314

Reference keys
NOMENCLATURE AND DESCRIPTION FOR RATING GUIDELINE RECOMMENDATIONS
Within each recommendation, the strength of recommendation is indicated as Level 1 or Level 2, and the certainty of the supporting
evidence is shown as A, B, C,orD.
Grade
Implications
Patients Clinicians Policy
Level 1
“We recommend”
Most people in your situation would
want the recommended course of
action, and only a small proportion
would not.
Most patients should receive the
recommended course of action.
The recommendation can be evaluated
as a candidate for developing a policy
or a performance measure.
Level 2
“We suggest”
The majority of people in your situation
would want the recommended course
of action, but many would not.
Different choices will be appropriate for
different patients. Each patient needs
help to arrive at a management
decision consistent with their values
and preferences.
The recommendation is likely to require
substantial debate and involvement of
stakeholders before policy can be
determined.
Grade Certainty of evidence Meaning
A High We are confident that the true effect is close to the estimate of the effect.
B Moderate The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
C Low The true effect may be substantially different from the estimate of the effect.
D Very low The estimate of effect is very uncertain, and often, it will be far from the true effect.
Practice points are consensus-based statements representing the expert judgment of the Work Group and are not graded. They are issued when a
clinical question did not have a systematic review performed, to help readers implement the guidance from graded recommendation (e.g., frequency
of monitoring, provision of standard care [such as regular clinic visits], referral to specialist care, etc.), or for issuing “good practice statements” when
the alternative is considered to be absurd. Users should consider the practice point as expert guidance and use it as they see fit to inform the care of
patients. Although these statements are developed based on a different methodology, they should not be seen as “less important” or a “downgrade”
from graded recommendations.
www.kidney-international.org reference keys
Kidney International (2024) 105 (Suppl 4S), S117–S314 S125

CURRENT CHRONIC KIDNEY DISEASE (CKD) NOMENCLATURE USED BY KDIGO
CKD is defined
as abnormalities of kidney structure or function, present for a minimum of 3 months, with implications for health. CKD is
classified
based on Cause, Glomerular filtration rate (GFR) category (G1–G5), and Albuminuria category (A1–A3), abbreviated as CGA.
Persistent albuminuria categories
Description and range
GFR categories (ml/min/1.73 m
2
)
Description and range
A1
G1
≥90
G2
60–89
G3a
45–59
G3b
30–44
G4
15–29
G5 <15Kidney failure
Severely decreased
Moderately to
severely decreased
Mildly to
moderately decreased
Mildly decreased
Normal or high
A2 A3
Normal to mildly
increased
Moderately
increased
Severely
increased
<30 mg/g
<3 mg/mmol
30–300 mg/g
3–30 mg/mmol
>300 mg/g
>30 mg/mmol
KDIGO: Prognosis of CKD by GFR
and albuminuria categories
Green: low risk (if no other markers of kidney disease, no CKD); Yellow: moderately increased risk; Orange: high
risk; Red: very high risk. GFR, glomerular filtration rate.
CKD nomenclature www.kidney-international.org
S126
Kidney International (2024) 105 (Suppl 4S), S117–S314

CONVERSION FACTORS OF CONVENTIONAL UNITS TO SI UNITS
Conventional unit Conversion factor SI unit
Albumin-to-creatinine ratio (ACR) mg/g 0.113 mg/mmol
Calcium mg/dl 0.2495 mmol/l
Creatinine mg/dl 88.4
m
mol/l
Protein-to-creatinine ratio (PCR) mg/g 0.113 mg/mmol
Phosphate mg/dl 0.3229 mmol/l
Urate mg/dl 59.48 mmol/l
SI, International System of Units.
Note: Conventional unit conversion factor ¼ SI unit.
EQUIVALENT ALBUMINURIA CATEGORIES IN CKD
Category AER (mg/24 h)
ACR (approximate equivalent)
Terms(mg/mmol) (mg/g)
A1 <30 <3 <30 Normal to mildly increased
A2 30–300 3–30 30–300 Moderately increased
a
A3 >300 >30 >300 Severely increased
ACR, albumin-creatinine ratio; AER, albumin excretion rate; CKD, chronic kidney disease.
a
Relative to the young adult level.
www.kidney-international.org conversion factors
Kidney International (2024) 105 (Suppl 4S), S117–S314 S127

Abbreviations and acronyms
ACEi angiotensin-converting enzyme inhibitor(s)
ACR albumin-to-creatinine ratio
ADA American Diabetes Association
ADPKD autosomal dominant polycystic kidney disease
AER albumin excretion rate
AIDS acquired immune de ficiency syndrome
AKD acute kidney disease
AKI acute kidney injury
ARB angiotensin II receptor blocker
ASCVD atherosclerotic cardiovascular disease
BMI body mass index
BP blood pressure
BSA body surface area
CI confidence interval
CKD chronic kidney disease
CKD-EPI Chronic Kidney Disease Epidemiology
Collaboration
CKiD Chronic Kidney Disease in Children
CKD-MBD chronic kidney disease-mineral and bone
disorder
CKD-PC Chronic Kidney Disease Prognosis
Consortium
CrCl creatinine clearance
CT computed tomography
CVD cardiovascular disease
DALY disability-adjusted life-year
eGFR estimated glomerular filtration rate
eGFRcr creatinine-based estimated glomerular
filtration rate
eGFRcr-cys creatinine and cystatin C–based estimated
glomerular filtration rate
eGFRcys cystatin C–based estimated glomerular
filtration rate
EKFC European Kidney Function Consortium
EMA European Medicines Agency
EMR electronic medical record
ERT Evidence Review Team
FDA Food and Drug Administration
GBD Global Burden of Disease
GFR glomerular filtration rate
GLP-1 RA glucagon-like peptide-1 receptor agonist(s)
GN glomerulonephritis
HBV hepatitis B virus
HCV hepatitis C virus
HDL high-density lipoprotein
HIV human immunodeficiency virus
HR hazard ratio
HRQoL health-related quality of life
IgG immunoglobulin G
IQR interquartile range
i.v. intravenous
KDIGO Kidney Disease: Improving Global
Outcomes
KDOQI Kidney Disease Outcomes Quality Initiative
KFRE Kidney Failure Risk Equation
KRT kidney replacement therapy
LDL low-density lipoprotein
LMIC low- and middle-income countries
MACE major adverse cardiovascular events
MDRD Modification of Diet in Renal Disease
mGFR measured glomerular filtration rate
MRA mineralocorticoid receptor antagonist(s)
mTOR mammalian target of rapamycin
NICE National Institute for Health and Care
Excellence
NIHR National Institute for Health and Care
Research
NOAC non–vitamin K antagonist oral
anticoagulant
NSAIDs nonsteroidal anti-inflammatory drugs
OR odds ratio
OTC over-the-counter
PCR protein-to-creatinine ratio
PCSK-9 proprotein convertase subtilisin/kexin type-9
PICOS population, intervention, comparator,
outcomes, study design
POCT point-of-care testing
PROM patient-reported outcome measure
QoL quality of life
RAS(i) renin-angiotensin system (inhibitor)
RAAS(i) renin-angiotensin-aldosterone system
(inhibitor)
RBC red blood cell
RCT randomized controlled trial
RR relative risk
SCr serum creatinine
SBP systolic blood pressure
SES socioeconomic status
SGLT2i sodium-glucose cotransporter-2
inhibitor(s)
T1D Type 1 diabetes
T2D Type 2 diabetes
UK United Kingdom
US United States
USRDS United States Renal Data System
WHO World Health Organization
abbreviations and acronyms www.kidney-international.org
S128
Kidney International (2024) 105 (Suppl 4S), S117–S314

Notice
SECTION I: USE OF THE CLINICAL PRACTICE GUIDELINE
This Clinical Practice Guideline document is based upon literature searches conducted from July 2022 throug h April 2023 and
updated in July 2023. It is designed to assist decision-making. It is not intended to define a standard of care and should not be
interpreted as prescribing an exclusive course of management. Variations in practice will inevitably and appropriat ely occur
when clinicians consider the needs of individual patients, available resources, and limitations unique to an institution or type of
practice. Healthcare providers using the statements in this document (both practice points and recommendations) should
decide how to apply them to their own clinical practice.
SECTION II: DISCLOSURE
Kidney Disease: Improving Global Outcomes (KDIGO) makes every effort to avoid any actual or reasonably perceived conflicts
of interest that may arise from an outside relationship or a personal, professional, or business interest of a member of the Work
Group. All members of the Work Group are required to complete, sign, and submit a disclosure and attestation form showing
all such relationships that might be perceived as or are actual conflicts of interest. This document is updated annually, and
information is adjusted accordingly. All reported information is published in its entirety at the end of this document in the
Work Group members’ Disclosure section and is kept on file at KDIGO.
Copyright 2023, Kidney Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the
International Society of Nephrology. This is an open access article under the CC BY-NC-ND license (http://
creativecommons.org/licenses/by-nc-nd/4.0/). Single copies may be made for personal use as allowed by national
copyright laws. Special rates are available for educational institutions that wish to make photocopies for nonprofit
educational use. No part of this publication may be reproduced, amended, or transmitted in any form or by any means,
electronic or mechanical, including photocopying, recording, or any information storage and retr ieval system, without
explicit permission in writing from KDIGO. Details on how to seek reprints, permission for reproduction or translation, and
further information about KDIGO’s permissions policies can be obtained by contacting Melissa Thompson, Chief Operating
Officer, at melissa.th ompson@kdigo.org.
Neither KDIGO, Kidney International, the Publisher, nor the authors, contributors, or editors shall have or assume any
liability for any direct, indirect, incidental, special, exemplary, or consequential damages (including without limitation lost
profits) or any injury and/or damage to persons or property, however caused and on any theory of liability, whether in
contract, strict liability, or tort (includi ng product liability, negligence or otherwise) arising in any way out of the use or
operation of any methods, products, instructions, or ideas contained in the material herein.
www.kidney-international.org notice
Kidney International (2024) 105 (Suppl 4S), S117–S314 S129

Foreword
Kidney International (2024) 105 (Suppl 4S), S117–S314; https://doi.org/10.1016/j.kint.2023.10.018
Copyright ª 2023, Kidney Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the International Society of Nephrology. This is an open access
article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
The Kidney Disease: Improving Global Outcomes (KDIGO)
organization was established in 2003 with the mission to
improve the care and outcomes of people living with kidney
disease worldwide. The development and implementation of
global clinical practice guidelines is central to the many ac-
tivities of KDIGO to fulfill its mission. Twenty years later, we
are excited to present this update of the KDIGO Clinical
Practice Guideline for the Evaluation and Management of
Chronic Kidney Disease (CKD) to complement the existing
12 guidelines that address various other facets of kidney
disease management.
Our aspiration is that the KDIGO CKD Guideline serves as
a comprehensive reference for evidence-based practices, of-
fering clear and valuable guidance for the optimal diagnosis
and treatment of CKD. The updated guideline is the result of
a rigorous process, extensively detailed in the KDIGO
Methods Manual. To promote objectivity and transparency,
we screen Guideline Co-Chairs and Work Group members
(which include clinicians, researchers, and patients) for con-
flicts of interest. Over a span of 2–3 years, these individuals
volunteer their time, starting with the creation of a Scope of
Work that undergoes an open public review to engage all
stakeholders. This docu ment is then adapted into a Request
for Proposal, which is used to enlist an independent Evidence
Review Team.
The Evidence Review Team conducts a systematic review of
existing literature, extracting studies with appropriate design
and outcomes deemed important by both people with CKD
and clinicians. All work is meticulously graded on study
quality and potential bias, forming the basis for quantifying
the overall certainty of the evidence using the “Grading of
Recommendations Assessment, Development, and Evalua-
tion” (GRADE) approach. The penultimate version of the
guideline also undergoes public review to capture additional
perspectives. Thus, guidelines are the result of a rigorous and
objective assessment of available evidence, enriched by the
collective expertise of healthcare providers, researchers, and
patients al ike. Guideline statements (“We recommend” or
“We suggest”)reflect clinical questions that were addressed by
the evidence reviews from the Evidence Review Team. Practice
points provide guidance on clinical questions that were not,
and largely could not be, studied by the Evidence Review
Team.
We view the current guideline as a dynamic, evolving
resource rather than a static document. We are delighted by the
recent pace of clinical discovery that substantially increased the
scientific basis of optimal CKD diagnosis and management,
and we remain committed to updating recommendations and
practice points as important evidence emerges. We hope that
the guideline will serve as a useful tool for clinicians in their
daily practice, prov iding clear insight into the evidence-based
recommendations while highlig hting areas requiring further
research. Ultimately, our aim is to facilitate more effective and
consistent care to patients with CKD worldwide, and the
publication of the CKD Guideline will provide the foundation
of many dissemination and implementation activities to in-
crease the outreach and usefulness of this work.
We extend our heartfelt g ratitude for all those who have
contributed to the CKD Guideline. First, to the members of
the Methods Committee, particularly Dr. Marcello Tonelli,
MD, SM, MSc, Chair of the Committee, and Amy Earley, BS,
KDIGO Guideline Development Director, for setting the
expectation of rigor, balance, and transparency throughout
the process. Next, to the Evidence Review Team at Johns
Hopkins University, for their meticulous work in reviewing
the existing literature. Third, to the Work Group members,
led by the indefatigable Drs. Adeera Levin, MD, and Paul
Stevens, MB, for their diligence and innumerable hours vol-
unteered to shepherd the guideline to publication. Fourth, to
the many individuals who provided comments during the
rounds of public revie w. Finally, to the whole KDIGO staff,
for their steadfast, behind the scenes commitment to excel-
lence in patient care.
Sincerely,
Morgan E. Grams, MD, PhD, MHS
Michel Jadoul, MD
KDIGO Co-Chairs
foreword www.kidney-international.org
S130
Kidney International (2024) 105 (Suppl 4S), S117–S314

Work Group membership
WORK GROUP CO-CHAIRS
Paul E. Stevens, MB, FRCP, RCPathME
East Kent Hospitals University
NHS Foundation Trust
Canterbury, United Kingdom
Adeera Levin, MD, FRCPC
University of British Columbia
Vancouver, Canada
WORK GROUP
Sofia B. Ahmed, MD, MMSc, FRCPC
University of Alberta
Edmonton, Alberta, Canada
Juan Jesus Carrero, Pharm, PhD Pharm, PhD Med,
MBA, FNKF, FERA
Karolinska Institutet
Stockholm, Sweden
Bethany Foster, MD, MSCE
McGill University
Montreal, Quebec, Canada
Anna Francis, MBBS, FRACP, CF, MMed, PhD
Queensland Children’s Hospital
Brisbane, Australia
Rasheeda K. Hall, MD, MBA, MHS
Duke School of Medicine
Durham, North Carolina, USA
Will G. Herrington, MA, MBBS, MD, FRCP
University of Oxford
Oxford, United Kingdom
Guy Hill
Manchester, United Kingdom
Lesley A. Inker, MD, MS, FRCP(C)
Tufts Medical Center
Boston, Massachusetts, USA
Rümeyza Kazancıo
glu, MD
Bezmialem Vakif University
Istanbul, Turkey
Edmund Lamb, PhD, FRCPath
East Kent Hospitals University NHS
Foundation Trust
Canterbury, United Kingdom
Peter Lin, MD, CCFP
Canadian Heart Research Center
Toronto, Ontar io, Canada
Magdalena Madero, MD
Instituto Nacional de Cardiología Ignacio Chavéz
Mexico City, Mexico
Natasha McIntyre, PhD
Western University
London Health Sciences Centre-Victoria Hospital
London, Ontario, Canada
Kelly Morrow, MS, RDN, CD, FAND
Basty r University, Osher Center for Integrative
Medicine University of Washington
Kenmore, Washington, USA
Glenda Roberts
UW Center for Dialysis Innovation &
Kidney Research Institute
Seattle, Washington, USA
Dharshana Sabanayagam, MD, FRACP
University of Sydney
Sydney, Australia
Elke Schaeffner, MD, MSc
Charité Universitätsmedizin Berlin
Berlin, Germany
Michael Shlipak, MD, MPH
University of California, San Francisco
San Francisco, California, USA
Rukshana Shroff, MD, FRCPCH, PhD
UCL Great Ormond Street Hospital Institute of Child Health,
London, United Kingdom
Navdeep Tangri, MD, PhD, FRCP(C)
University of Manitoba
Winnipeg, Manitoba, Canada
Teerawat Thanachayanont, MD, MSc
Bhumirajanagarindra Kidney Institute
Bangkok, Thailand
Ifeoma Ulasi, MBBS, FWACP, PGD, MSc
University of Nigeria, Ituku-Ozalla Campus
Enugu, Nigeria
www.kidney-international.org Work Group membership
Kidney International (2024) 105 (Suppl 4S), S117–S314 S131

Germaine Wong, MD, PhD
University of Sydney
Sydney, Australia
Chih-Wei Yang, MD
Chang Gung University
Taoyuan, Taiwan
Luxia Zhang, MD, MPH
Peking University First Hospital
Beijing, China
METHODS COMMITTEE REPRESENTATIVE
Bertram L. Kasiske, MD, FACP
Hennepin County Medical Center
University of Minnesota
Minneapolis, MN, USA
EVIDENCE REVIEW TEAM
The Johns Hopkins University Evidence-based Practice Center
Karen A. Robinson, PhD, Professor of Medicine
Lisa Wilson, ScM, Research Associate
Renee F. Wilson, MS, Research Associa te
Dipal M. Patel, MD, PhD, Assistant Professor of Medicine
Troy Gharibani, BS, BA, Research Assistant
Xuhao Yang, MSPH, Research Assistant
Verna Laz ar, MBBS, MPH, Research Assistant
Jeongmin Hana Kim, PharmD, MSc, Research Assistant
Work Group membership www.kidney-international.org
S132
Kidney International (2024) 105 (Suppl 4S), S117–S314

Abstract
The Kidney Disease: Improving Global Outcomes (KDIGO) 2024 Clinical Practice Guideline for
the Evaluation and Management of Chronic Kidney Disease (CKD) is an update to the KDIGO
2012 guideline on the topic. The aim is to assist clinicians caring for people with CKD, both
adults and children. People receiving dialysis and kidney transplant recipients are not the focus of
this guideline. The scope includes chapters dedicated to the evaluation of CKD, risk assessment in
people with CKD, manage ment to delay CKD progression and manage its complications, medical
management and drug stewardship in CKD, and optimal models of CKD care. In addition, this
guideline includes a comprehensive introduction from the guideline Co-Chairs, a patient
foreword, a discussion of special population considerations, a presentation of the relative and
absolute risks associated with specific outcomes from the CKD Prognosis Consortium (CKD-
PC), and an extensive section dedicated to research recommendations based on the current gaps
in evidence. The goal of the guideline is to generate a useful resource for clinicians and patients by
providing actionable recommendations based on a rigorous formal evidence review, practice
points that serve to direct clinical care or activities for which a systematic review was not con-
ducted, and useful infographics. The guideline targets a broad audience of healthcare providers
involved in the care of people with CKD as well as people with CKD themselves while being
mindful of implications for policy and payment. Development of this guideline update followed
an explicit process of evidence review and appraisal. Treatment approaches and guideline rec-
ommendations are based on systematic reviews of relevant studies, and appraisal of the certainty
of the evidence and the strength of recommendations followed the “Grading of Recommenda-
tions Assessment, Development, and Evaluation” (GRADE) approach. Limitations of the evi-
dence are discussed, with areas of future research also presented.
Keywords: chronic kidney disease; CKD; evaluation; guideline; KDIGO; management
CITATION
In citing this document, the following format should be used: Kidney Diseas e: Improving
Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical Practice Guideline for
the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):
S117–S314.
www.kidney-international.org abstract
Kidney International (2024) 105 (Suppl 4S), S117–S314 S133

Patient foreword
The identification of chronic kidney disease (CKD) begins a
long journey for any patient that will have a direct impact on
their lifestyle and future health outcomes. This guideline
identifies the suitability of medical interventions that can
improve or delay the ser iousness of CKD and possible kidney
failure.
In a complicated world of health provision, having a set of
evidential recommendations and practice points provides
kidney service providers with the targets for a quality CKD
service for people with kidney disease. However, if the starting
point for many people is ignorance of what a kidney actually
does, then without a holistic approach to patient care, much of
the potential effectiveness of medical interventions can be
diluted because of patient circumstances and psychological
challenges.
Acceptance of the seriousness of CKD can take a lot
longer for a person to process, to the possible detr iment of
medical intervention, and may well lead to issues over
adherence.
A controlled, managed CKD decline is so beneficial to
patients who have so many social issues to contend with, be it
diet, tiredness, liquid control, pill overload, and a deep dive
into the very mechanics of how we eat and drink to survive
and excrete excesses.
In an ever-increasingly busy world of medical care, as pa-
tients, we believe that the best approach is for any physician to
aim to achieve a partnership of knowledge with the patient
regarding their CKD care. This will build patient confidence and
self-awareness, with the aim that any patient who sadly arrives at
possible dialysis is in the right state of mind, which is critical for a
considered approach to the next stage of a patient’s journey.
Guy Hill
CKD Work Group Member
patient foreword www.kidney-international.org
S134
Kidney International (2024) 105 (Suppl 4S), S117–S314

Introduction, qualifying statements, and key concepts
This 2024 update of the KDIGO Clinical Practice Guideline
for the Evaluation and Management of Chronic Kidney Dis-
ease (CKD)
1
is an evidence-based guideline that provides
recommendations and practice points for clinical
management activities.
The past 10 years have provided new hope for improved
treatment of CKD. A greater under standing of healthy lifestyle
and lifestyle modifications together with new medications
and technologies furnish improved options for treatment and
monitoring of CKD. People with CKD, healthcare providers,
and health systems are eager to implement these advances in
the most effective and evidence-based manner. This requires
integration of new therapies with lifestyle management and
existing medications using approaches that engage patients
and optimize application of health resources. The goal of this
guideline document is to provide such guidance. The majority
of statements from the 2012 guideline have been updated
based on current knowledge and practice. Only 6 statements
were retained in their original form in 2012.
As Co-Chairs, we would like to recognize the outstanding
efforts of the Work Group, the Evidence Review Team (ERT),
and Kidney Disease: Improving Global Outcomes (KDIGO)
staff. The Work Group was diverse, multinational, multidis-
ciplinary, experienced, thoughtful, and dedicated. Notably,
the Work Group included 2 members who have CKD who
contributed actively as peers to keep the guideline relevant
and patient-centered. We are indebted to each and every in-
dividual who contributed to this process. We hope that the
guidance provided here will help improve the care of people
with CKD worldwide.
The KDIGO 2012 CKD guideline was built on the United
States (US)-based Kidney Disease Outcomes Quality Initiative
(KDOQI) 2002 Guideline on Definition, Classification, and
Evaluation of CKD,
2
accepted by the international
community in 2005. It reinforced the definition of CKD
incorporating a persistent reduction in glomerular filtration
rate (GFR) and markers of kidney damage and modified the
staging and classification system to include elements that
had begun to be appreciated by the clinical community.
3
Specifically, the 2012 guideline introduced the concept of a
“CGA” classification of CKD based on cau se (C), level of
kidney function determined by GFR (G), and degree of
albuminuria (A). The CGA classification laid a foundation
upon which management, treatment, research, and risk
assessment of CKD have since been based.
The definition, staging, and classification of CKD pro-
posed by the KDIGO 2012 CKD guideline have been widely
accepted and implemented worldwide. Research has since
highlighted that higher specific stages or categories of CKD,
characterized by level of GFR and albuminuria independently,
portend greater relative risk (RR) for adverse outcomes.
4–7
These include, but are not limited to, CKD progression,
cardiovascular disease (CVD), mortality (all-cause and car-
diovascular), kidney failure, and acute kidney injury (AKI).
The development of risk-prediction tools has refined moni-
toring and referral to specialist nephrology and has aided in
the estimation of prognosis.
6,8–10
Although there remains
ongoing discussion about appli cation of the same thresholds
to define disease in older adults,
11
it is still clear that even
in older populations, risk of adverse outcomes increases
with higher CKD stages (Figure 1).
12
In any field of
medicine, although data from large population studies
inform clinical practice guidelines and associated
recommendations for care, it is critically important to
consider the individual in front of you, their preferences,
and their individual risks and benefits. We recognize that
the threshold GFR <60 ml/min per 1.73 m
2
(GFR
categories G3a–G5) for >3 months to indicate a diagnosis
of CKD is well below the average in young adult men and
women,
13
but because a significant GFR reduction in
younger people is also usually associated w ith other markers
of kidney disease, the diagnosis of CKD would be captured.
Similarly, we recognize that there is an average age-
associated GFR decline observed in longitudinal and cross-
sectional studies,
14
but with substantial variation amon g
individuals within the population,
15
such that not all
individuals will have a significant GFR decline with age.
12
This guideline is not intended to be a textbook, and thus
statements regarding prevention and screening for CKD,
although important topics, are not addressed in depth but are
briefly discussed below in the context of the global burden of
CKD and in Chapter 1. For a more detailed discussion of these
issues, we refer readers to existing textbooks and reviews.
16–18
Prevention and screening for CKD should be conducted
mostly by healthcare providers in primary care and in other
specialties, such as endocrinology, cardiology, and oncology,
rather than restricted to nephrologists. We strongly support
efforts aimed at the early detection and treatment of CKD
among people at high risk for CKD, including those with
hypertension, diabetes, and CVD. Screening efforts in these
and other populations should include assessments of GFR
(estimated or in certain situations measured [see Section 1.2]
and albuminuria [or surrogate, see Section 1.3]).
The intended starting point for this update of the KDIGO
2012 CKD guideline is an established diagnosis of CKD,
though there are some practice points to clarify the evaluation
of CKD and the ascertainment of chronicity. The care of
people with CKD is multifaceted and complex. Several critical
aspects of this comprehensive care, such as blood pressure
(BP), diabetes, and lipid management, have been addressed in
other KDIGO guidelines. These topics were not reviewed for
the current guideline, but recommendations have been
incorporated where relevant and we refer readers to those
specific KDIGO guidelines and their updates.
19–23
www.kidney-international.org introduction, qualifying statements, and key concepts
Kidney International (2024) 105 (Suppl 4S), S117–S314 S135
This clinical practice guideline includes 2 different types of
statements: graded recommendations, which are supported by
systematic reviews (i.e., de novo reviews conducted by the in-
dependent ERT or existing high-quality reviews that have been
systematically identified), and ungraded practice points, which
serve to direct clinical care or activities for which a systematic
review was not conducted for various reasons (e.g., lack of a
sufficient evidence base or randomized controlled trials
[RCTs] would be impractical/unethical). Both recommenda-
tions and practice points are intended to help guide clinical
practice and aid in decision-making; thus, they collectively are
the guidance statements. They are clearly articulated and
presented together so that all guideline statements can be
implemented. The distinction between them is based on the
process by which they are derived, that process is based on the
framework methodology from the KDIGO Methods Com-
mittee and aligns with other international guideline groups
utilizing the “Grading of Recommendations Assessment,
Development, and Evaluation” (GRADE) methodology.
Several exciting developments have been introduced into
clinical practice since the KDIGO 2012 CKD guideline was
published. These include refinement of evaluation of GFR,
population and individual risk prediction, and novel treat-
ments which have all positively influenced the prognosis for
people with CKD. The Work Group has aimed to generate a
guideline that is both rigorously devoted to new and existing
evidence, and clinically useful.
Research recommendations are presented in a separate
section at the end of this document and are intended to guide
the next set of important research questions to inform and
improve outcomes of people living with CKD. The research
recommendations are not exhaustive but are intended to help
focus the clinical and research communities on unanswered
questions including improving diagnostic tools and evalua-
tion of kidney function, development and testing of risk
prediction equations in clinical and research settings, evalu-
ation of different therapies to delay progression in various
combinations, improved medication management, and
optimal models of care. We specifically urge the community
to be in clusive of people across the lifecycle and include sex
and ge nder, and etiology of CKD, as important variables in all
studies.
Age <65
eGFRcr-cys <10 10–29 30–299 300+ <10 10–29 30–299 300+
105+ 0.99 1.2 1.5 2.4 0.93 1.0 1.1 2.6
90–104 ref 1.3 1.5 2.5 ref 1.2 1.3 1.9
60–89 1.2 1.6 2.0 2.9 1.3 1.4 1.6 2.1
45–59 2.1 2.7 2.9 4.5 1.8 2.6 3.1 3.5
30–44 2.7 3.8 4.2 5.6 1.9 2.3 3.0 3.9
<30 5.2 4.0 7.1 8.6 4.1 3.6 4.7 5.8
105+ 0.95 1.4 1.7 4 0.96 1.2 1.6 2.7
90–104 ref 1.6 1.8 3.5 ref 1.2 1.5 2.2
60–89 1.3 1.7 2.3 3.9 1.2 1.4 1.7 2.6
45–59 2.5 4.0 4.6 6.0 1.9 2.0 2.5 3.8
30–44 3.1 6.6 5.3 7.1 2.6 3.7 3.5 3.5
<30 6.0 5.5 9.4 12 2.6 2.9 5.1 5.1
105+ 0.57 0.77 2.3 12 0.86 1.1 1.7 3.4
90–104 ref 1.4 3.9 11 ref 1.3 1.5 3.0
60–89 1.9 3.7 8.3 33 1.2 1.7 2.1 3.6
45–59 7.0 16 28 100 1.7 3.3 3.4 5.3
30–44 22 34 109 210 3.5 4.3 6.8 5.7
<30 335 267 419 625 7.5 6.3 9.7 8.9
105+ 0.75 1.0 1.4 3.4 0.93 1.0 1.3 1.9
90–104 ref 1.2 1.8 2.6 ref 1.2 1.4 2.3
60–89 1.6 2.7 2.9 5.8 1.1 1.3 1.5 1.8
45–59 4.2 6.0 5.6 7.6 1.5 2.0 2.1 2.6
30–44 5.7 9.4 9.8 9.4 1.8 2.4 3.0 2.8
<30 15 14 14 13 3.7 2.9 4.3 5.4
105+ 1.0 1.1 1.1 1.5 0.93 1.9 1.5 2.6
90–104 ref 1.1 1.2 1.3 ref 1.8 2.1 3.9
60–89 1.1 1.2 1.3 1.6 1.2 2.1 2.2 5.4
45–59 1.3 1.7 1.5 2.0 3.2 7.3 3.4 8.4
30–44 1.5 1.8 1.6 2.1 6.5 9.1 6.6 13
<30 2.1
2.4 2.4 3.5 1.4 7.6 18 16
esaesid yretra larehpirePnoitazilatipsoH
Kidney failure replacement therapy Heart failure
ekortSytilatrom ralucsavoidraC
noitcrafni laidracoyMytilatrom esuac-llA
+56 egAg/gm ,RCAg/gm ,RCA
eGFRcr-cys <10 10–29 30–299 300+ <10 10–29 30–299
105+ 1.2 1.4 1.9 3.5 0.97 1.4 2.0
90–104 ref 1.2 1.4 2.0 ref
1.2 1.1
60–89 1.2 1.5 1.8 2.3 1.1 1.4 1.5
45–59 1.6 2.0 2.4 2.9 1.6 1.9 2.3
30–44 2.0 2.4 3.2 4.1 2.1 2.6 3.1
<30 3.4 4.1 5.1 6.5 4.9 3.0 5.1
105+ 1.1 1.5 2.0 12 1.2 1.3 1.5
90–104 ref 1.4 1.4 3.4 ref 1.3 1.3
60–89 1.2 1.7 2.2 3.1 1.1 1.4 1.8
45–59 1.7 2.4 3.0 4.3 1.5 1.7 2.0
30–44 2.4 3.1 4.5 5.8 1.5 2.0 2.1
<30 5.7 5.2 5.1 7.8 1.7 2.0 2.4
105+ 2.0 1.0 2.1 0.99 1.5 1.7
90–104 ref 1.9 4.7 10 ref 1.3 1.5
60–89 1.4 2.6 6.2 19 1.2 1.5 2.0
45–59 3.7 7.9 16 42 1.6 2.0 2.9
30–44 14 14 46 137 2.3 2.9 3.5
<30 87 364 241 406 4.4 4.1 5.5
105+ 0.91 1.1 1.3 1.9 0.95 1.1 1.0
90–104 ref 1.3 1.4 3.9 ref 1.2 1.3
60–89 1.5 2.1 2.7 4.7 1.1 1.2 1.5
45–59 3.6 4.3 5.1 7.3 1.2 1.4 1.7
30–44 5.7 5.9 7.2 9.8 1.5 1.8 2.0
<30 10 11 11 22 1.8 1.8 2.2
105+ 1.0 1.1 1.2 2.2 1.1 2.3 2.9
90–104 ref 1.1 1.3 1.4 ref 1.3 2.0
60–89 1.1 1.2 1.3 1.5 1.3 1.6 2.0
45–59 1.2 1.2 1.4 1.6 2.0 2.8 3.1
30–44 1.5 1.4 1.6 2.0 3.5 2.8 3.8
<30 1.9 1.9 2.0 2.6 8.4 4.1 5.9
esaesid yretra larehpirePnoitazilatipsoH
Kidney failure replacement therapy Heart failure
ekortSytilatrom ralucsavoidraC
noitcrafni laidracoyMytilatrom esuac-llA
g/gm ,RCAg/gm ,RCA
300+
19
1.9
1.9
3.4
3.8
5.0
3.3
2.8
2.5
2.3
2.3
4.8
7.0
2.2
3.2
4.1
6.1
7.2
3.7
2.4
2.0
1.9
2.2
3.2
4.9
4.8
3.2
3.1
5.9
10
Figure 1 | Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by creatinine and cystatin C
(eGFRcr-cys) and albumin-to-creatinine ratio (ACR) categories and risks for 10 common complications by age in multivariable-adjusted
analyses. Numbers reflect the adjusted hazard ratio compared with the reference cell. Adjustment variables included age, sex, smoking status
(current, former, or never), systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, use of
antihypertensive medications, and a medical history of diabetes, coronary heart disease, stroke, heart failure, atrial fibrillation, peripheral artery
disease, cancer, and chronic obstructive pulmonary disease, where relevant. The colors were determined for each outcome separately using the
following rule: the percentile shaded the darkest green color corresponds to the proportion of cells in the grid without CKD (e.g., 6 of 24 cells),
and the percentile shaded the darkest red color corresponds to proportion expected to be at highest risk (e.g., 5 of 24 cells). In this manner, the
numbers of green and red cells are consistent across outcomes, but the patterns are allowed to differ. ref, reference cell. Reproduced with
permission from JAMA, Writing Group for the CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration
rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330(13):1266–1277.
12
Copyright ª 2023
American Medical Association. All rights reserved.
introduction, qualifying statements, and key concepts www.kidney-international.org
S136
Kidney International (2024) 105 (Suppl 4S), S117–S314

Definition and classification of CKD
Defining CKD.
CKD is defined as abnormalities of kidney
structure or function, present for a minimum of 3 months,
with implications for health (Table 1).
1
Classifying CKD. CKD is classified based on Cause, GFR
category (G1–G5), and Albuminuria category (A1–A3),
abbreviated as CGA.
1
These 3 components of the
classification system are each critical in the asse ssment of
people wit h CKD and help enable determination of severity
and risk. Listed below are reference tables describing each
component. Note that while the definition of CKD includes
many different markers of kidney damage and is not
confined to decreased GFR and albumin-to-creatinine ratio
(ACR) >30 mg/g [>3 mg/mmol], the classification system
is based on the 2 dimensions of GFR and degree of
albuminuria (Tables 2 and 3). This nuance is often missed
by healthcare providers and students.
It is well established that patient advocates with CKD and
healthcare providers prefer the more clinically useful and
generally understood assessment of GFR resulting from the
use of GFR estimating equations compared with serum
creatinine (SCr) alone. Globally, although still not universally
available in all countries, SCr is measured routinely and the
approach to assessment of GFR is therefore to use SCr and an
estimating equation for initial assessment of GFR. The
approach to evaluation of GFR using initial and supportive
tests is described in greater detail in Chapter 1.
Etiology of CKD should be sought, and there are
numerous systems fo r grouping various etiologies, some of
which are evolving with new knowledge and diagnostic tools.
There are congenital and genetic causes of CKD, some asso-
ciated with systemic diseases, and others that are primary. It is
beyond our remit to suggest a specific approach, but we
highlight the importance of establishing a cause to individ-
ualize management of CKD.
The global burden of CKD
The Global Burden of Disease, Injuries, and Risk Factors
Study (GBD) pulls together data on premature death and
disability from more than 350 diseases and injuries in 204
countries, by age and sex, from 1990 to the present.
24
Disease
“burden” is the impact of a health problem as measured by
financial cost, mortalit y, morbidity, or other indicators and
can be measured by combining 2 indicators to descr ibe the
disability-adjusted life-years (DALYs): the number of years
of life lost to disease and the number of years lived with
disability due to disease.
Globally, in 2017, a systematic analysis from the all-age
GBD project found 697.5 million (95% uncertainty interval
[UI]: 649.2–752.0) cases of all-stage CKD, for a global preva-
lence of 9.1% (8.5%–9.8%).
25
By 2021, a joint statement from
the American Society of Nephrology, European Renal
Association, and International Society of Nephrology
indicated that more than 850 million people suffer from
some form of kidney disease, roughly double the number of
people who live with diabetes (422 million) and 20 times
more than the prevalence of cancer worldwide (42 million)
or people living with AIDS/HIV (36.7 million). These
estimates derive from aggregation of studies worldwide,
which have applied a variety of definitions of CKD;
nevertheless, they furnish the best guide about global CKD
prevalence.
In 2017, CKD was estimated to account for 35.8 million
(95% UI: 33.7e38.0) DALYs, and 1.2 million people died
from CKD. Most of the burden of CKD was concentrated in
the 3 lowest quintiles of sociodemographic index (SDI). In
2019, CKD was responsible for 41.5 million (95% UI: 38.3–
45.0) DALYs, and 1.43 million people died from CKD.
24
Age-
standardized DALY rates (Figure 2
24
) were highest in central
and Andean Latin America, at 1348.1 (1203.6–1521.6) and
836.3 (704.2–981 .6) per 100,000, respectively (global rate
was 514.9 [474.9–558.9]). In 2017, CKD in diabetes
represented a third of all DALYs, and there were 1.4 million
Table 1 | Criteria for chronic kidney disease (either of the
following present for a minimum of 3 months)
Markers of kidney
damage (1 or more)
Albuminuria (ACR $30 mg/g [$3 mg/mmol])
Urine sediment abnormalities
Persistent hematuria
Electrolyte and other abnormalities due to
tubular disorders
Abnormalities detected by histology
Structural abnormalities detected by imaging
History of kidney transplantation
Decreased GFR GFR <60 ml/min per 1.73 m
2
(GFR categories G3a–G5)
ACR, albumin-to-creatinine ratio; GFR, glomerular filtration rate.
Table 2 | GFR categories in CKD
GFR
category
GFR (ml/min
per 1.73 m
2
) Terms
G1 $90 Normal or high
G2 60–89 Mildly decreased
a
G3a 45–59 Mildly to moderately decreased
G3b 30–44 Moderately to severely decreased
G4 15–29 Severely decreased
G5 <15 Kidney failure
CKD, chronic kidney disease; GFR, glomerular filtration rate.
a
Relative to the young adult level. In the absence of evidence of kidney damage,
neither G1 nor G2 fulfills the criteria for CKD.
Table 3 | Albuminuria categories in chronic kidney disease
Category
AER
(mg/24 h)
ACR (approximately
equivalent)
Terms(mg/mmol) (mg/g)
A1 <30 <3 <30 Normal to mildly
increased
A2 30–300 3–30 30–300 Moderately increased
a
A3 >300 >30 >300 Severely increased
ACR, albumin-to-creatinine ratio; AER, albumin excretion rate.
a
Relative to the young adult level.
www.kidney-international.org introduction, qualifying statements, and key concepts
Kidney International (2024) 105 (Suppl 4S), S117–S314 S137

(95% UI: 1.2–1.6) CVD-related deaths in peop le with CKD;
25.3 (22.2–28.9) million CVD DALYs were attributable to
impaired kidney function. Overall, CKD and its effect on
CVD resulted in 2.6 million (95% UI: 2.4–2.8) deaths in
2017 and CKD has risen from 19th to 11th in rank among
leading causes of death between 1990 and 2019 due to
aging and an increasing burden of risk factors for CKD
(including diabetes and hypertension) that, together,
contribute to more than half of the deaths from CKD.
Screening and prevention
Despite the increasing recognition of the true burden of CKD,
there remains controversy and lack of consensus as to the
utility of population screening for CKD
26
or targeted
screening programs,
18
due to the complexity of the
underlying sociopolitical and resource environment. Public
health policy has a role to play in identifying and
addressing risk factors to prevent CKD, to identify CKD
early, and to delay its progression and associated adverse
outcomes. Education of both health personnel and the
populations at risk, implementation of early kidney disease
detection programs, and incorporation of evidence-based
treatment of CKD and its associated conditions, such as BP
and diabetes, are all essential components of a strategy to
address this burden. A systematic review suggested that
screening for CKD is cost-effective in people with diabetes
and hypertension, the 2 most common causes of CKD
worldwide.
16
However, clinical trials have not been
conducted to determine whether or not an intervention to
detect, risk-stratify, and treat CKD would improve the
health outcomes for the targeted population. Nevertheless,
cost-effective analysis of population-wide screening for CKD
incorporating evidence-based treatment with sodium-
glucose cotransporter-2 inhibitors (SGLT2i) recently
concluded that screening adults for albuminuria to identify
CKD could be cost-effective in the United States.
27
This evidence aligns with the KDIGO Controversies Con-
ference on Early Detection and Intervention in CKD, which
concluded that early identification of CKD in people at risk,
who are usually asymptomatic, would likely be beneficial in the
community and primary care settings if the programs are
interwoven with risk stratification and treatment.
17
A
community program must be able to provide treatment to
the high-risk group of patients with newly detected CKD to
justify systematic early detection strategies. An additional
conclusion was that screening and treatment programs for
CKD should be implemented based on risk stratification to
prioritize people, particularly in settings with limited
economic resources. Although globally people with
hypertension, diabetes, or CVD are at high risk for CKD,
other high-risk people may be identified through genetic risk
factors or by varying exposure to environmental pollution,
pesticides, water, and nephrotoxic medications including
significant analgesic use and herbal medications, depending
on geographical region. Frameworks in which to consider
specific regional factors have been offered to facilitate
discussion about the value and context of screening for CKD.
26
Currently, kidney disease awareness remains low, and
worldwide only 6% of the general population and 10% of the
high-risk population are aware of their CKD status. Impor-
tant to note is that patient advocates with CKD strongly argue
for earlier CKD screening and diagnosis.
17
They also advocate
for CKD detection to be integrated with patient and family
education and engagement to improve accessing appropriate
healthcare and knowledge and adherence to recommended
lifestyle modification and medications.
0
500
1000
1500
2000
2500
0 10203040 60708090 00105
SDI
DALY rate (per 100,000)
Latin America and Caribbean
Central Europe, eastern Europe, and central Asia
Sub-Saharan Africa
High income
North Africa and Middle East
South Asia Southeast Asia, east Asia, and Oceania
GBD super-region
Figure 2 | Age-standardized chronic kidney disease disability-adjusted life-year (DALY) rates for each location by sociodemographic
index (SDI), both sexes combined, 2019. GBD, global burden of disease. Reproduced from Global Burden of Disease 2019: GBD cause and risk
summaries chronic kidney disease. Lancet. 2020;396:S152–S153.
24
ª 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article
under the CC BY 4.0 license.
introduction, qualifying statements, and key concepts www.kidney-international.org
S138
Kidney International (2024) 105 (Suppl 4S), S117–S314

Use of a simple algorithm such as that shown above in
settings such as primary care, cardiology, and endocrinology
could significantly improve the early identification and
treatment of CKD (Figure 3).
28
There are no current evidence-based recommendations
regarding the frequency of screening in people at risk of CKD.
In the setting of diabetes, a consensus report from the Amer-
ican Diabetes Association (ADA) and KDIGO recommends
annual screening of people with diabetes for CKD.
29
CKD
screening should start at diagnosis of type 2 diabetes (T2D)
because evidence of CKD is often already apparent at this
time. For type 1 diabetes (T1D), screening is recommended
commencing 5 years after diagnosis. The overall costs of a
screening program are largely driven by the frequency of
repeat screening, so the timing of repeated testing should be
guided by CKD risk. There are risk equations available to
estimate the interval risk of developing CKD, and this risk
stratification could guide repeat testing intervals.
30
International considerations
In low- and middle-income regions of the world and in the
lower sociodemographic quintiles, there is a large gap between
CKD burden and provision of adequate healthcare. There is
limited access to kidney replacement therapy (KRT) combined
with the rising prevalence of diabetes and hypertension and
evidence of substantial sex and gender disparities in access to
CKD treatment. These factors highlight the importance of early
identification and treatment of risk factors in primary care.
However, the majority of the world’s population with CKD is
in low- and middle-income countries (LMIC) where there are
disparities in access to laboratory diagnostic services, kidney
biopsy, and imaging services, in availability of appropriately
skilled healthcare providers and the availability and afford-
ability of medications. The International Society of Nephrology
survey assessing global kidney healthcare resources reported
that fewer than 1 in 4 surveyed countries had facilities available
for routine measurements of SCr or proteinuria.
31
Identify adults at risk for CKD
Test for GFR* and ACR ± other markers of kidney damage
†
Test for GFR or ACR if not performed and exclude AKI/AKD
AKI/AKD present:
follow AKI/AKD guidance
GFR <60 ml/min per 1.73 m
2
and/or
ACR ≥30 mg/g [3 mg/mmol] after 3 months
or earlier if evidence of chronicity
Measure eGFRcr-cys if not
performed and available
CKD not present
Timing of retesting based on
individual characteristics such
as risk of progression
Stage according to GFR and ACR
Establish underlying cause
Estimate risk of progression
Initiate treatment
GFR ≥60 ml/min per 1.73 m
2
and
ACR <30 mg/g [3 mg/mmol]
and no other markers of
kidney damage present
GFR <60 ml/min per 1.73 m
2
or ACR ≥30 mg/g [3 mg/mmol]
and/or other markers of kidney damage present
Figure 3 | Screening algorithm for diagnosis and staging of chronic kidney disease (CKD) in adults. Risk factor conditions are listed in
Table 5. *For recommended methods to estimate glomerular filtration rate (eGFR), see Section 1.2.
†
Markers of kidney damage other than
albuminuria may also be used to diagnose CKD, but albumin-to-creatinine ratio (ACR) and GFR are still required to determine stage and
estimate risk of progression. Acute kidney disease (AKD) is defined by the abnormalities of kidney function and/or structure with implications
for health and with a duration of #3 months.
28
The orange boxes indicate actions in people at risk for CKD and in whom testing should be
performed. The blue boxes indicate testing steps. The green boxes indicate the identification of CKD and its stages and the initiation of
treatment. The purple box indicates the identification of AKD/acute kidney injury (AKI). Please also see the Kidney Disease: Improving Global
Outcomes (KDIGO) Clinical Practice Guideline for Acute Kidney Injury.
97
www.kidney-international.org introduction, qualifying statements, and key concepts
Kidney International (2024) 105 (Suppl 4S), S117–S314 S139

Importantly, slowing CKD progression at early stages
should provide economic benefits and prevent the develop-
ment of kidney failure and cardiovascular complications. A
systematic review of care models in LMIC found that those
supporting primary care providers or allied health workers
achieved effectiveness in slowing GFR decline, as opposed to
interventions centered on specialty care alone.
32
Where there
are resource limitations, it is logical to deploy resources where
they w ill be most cost-effective, for example, to higher-risk,
preventable stages.
Standardization/accuracy of testing tools including
assays/equipment
The KDIGO 2012 CKD guideline built on recommendations
made to clinical laboratories in the earlier KDOQI 2002
guidance. Clinical laboratories were specifically charged with
measuring SCr and serum cystatin C using assays with cali-
bration traceable to the international standard reference
materials recommending that, for SCr, there should be
minimal bias compared with isotope-dilution mass spec-
trometry.
1
Recommendations were also made with respect to
measurement and reporting of albumin and protein in the
urine. Although some of the recommendations have
become part of routine practice, the effective use of clinical
guidelines and therefore effective patient care, including
accurate diagnosis and referral prioritization, clinical
research, and public health prioritization, require
comparability of laboratory results independent of time,
place, and measurement procedure. Key to this is
establishing precision and between-laboratory agreement
with traceability to accepted reference standards wherever
available. Therefore, this guidance document includes
standards for laboratory tests. The International
Consortium for Harmonization of Clinical Laboratory
Results (ICHLR) was established to create a pathway for
harmonization and aid implementation of clinical guidelines
recommending the use of laboratory tests in the diagnosis
and management of disease,
33
ensuring that both reference
materials and test methodology are harmonized. The
ICHLR aimed to prioritize measurands by medical
importance and both coordinate and stimulate development
of technical and regulatory processes to achieve
harmonization of those measuran ds.
34
Although this has
been achieved for SCr, the current status of other key
measurands such as cystatin C and urinary albumin is not
yet sufficiently clear.
The foundations for this 2024 guideline have been devel-
oped over the last 20 years, galvanizing the collaborative work
of researchers, healthcare providers, laboratory physicians,
patients, and carers. The current updated guideline document
reinforces methods for accurate diagnosis of CKD and pre-
diction, incor porates novel treatment strategies and ap-
proaches to managing people living with CKD, and identifies
further areas for research. Importantly, as the field is rapidly
changing, we commit to updating relevant sections of this
document as new evidence becomes available, to ensure more
timely updates than have previously been poss ible.
Adeera Levin, MD, FRCPC
Paul E. Stevens, MB, FRCP
CKD Guideline Co-Chairs
introduction, qualifying statements, and key concepts www.kidney-international.org
S140
Kidney International (2024) 105 (Suppl 4S), S117–S314

Special considerations
The Work Group recognizes that kidney diseases affect people
at different times and with different impacts across the whole
lifespan. Thus, enabling a personalized approach, considering
age, sex, and gender for diagnosis, risk assessment, and
treatment is critical. At the extremes of age—the very young
and the very old—diagnostic procedures, treatment aims,
treatment modalities, and decision-making differ due to dif-
ferences in prognosis, treatment options, and prioritization.
In young and middle-aged adults, treatment approaches may
differ due to specific circumstances, such as pregnancy or
menopause. Sex (biological attributes) and gender (socio-
cultural factors), as well as other important intersectional
factors including but not limited to geographical location,
socioeconomic status (SES), and race and ethnicity, play
important roles in kidney health and disease.
Here we introduce concepts as to why age, sex, and gender
should be considered in the context of diagnosis, treatment,
and care planning in people with CKD. In addition, the
specific guideline chapters incorporate statements where
special considerations regarding age, sex, and gender are
relevant to clinical practice and understanding.
Considerations in children and adolescents
When the guideline refers to people with CKD, this includes
children (people <10 years old) and adolescents (people
10–19 years old). When there are altered care recommenda-
tions and practice points due to the unique needs of children
or the lack of data to inform recommendations and practice
points, these considerations are discussed w ithin the Pediatric
considerations sections of the guideline.
The management of children and adolescents with CKD
needs special consideration (Figure 4). Children and adults have
different etiologies of CKD. Up to 40%–50% of childhood CKD
is due to congenital anomalies of the kidneys and urinary tract
(CAKUT); the younger the CKD population, the greater the
proportion with CAKUT as the cause.
35,36
CAKUT is
characterized by slower progression to kidney failure and a
higher likelihood of polyuria than the conditions causing CKD
in adults. Pediatric CKD has several unique aspects:
Delivery of care. Pediatric healt hcare providers engage with
not only the person with CKD but also their carers and sib-
lings. Age-appropriate care and education, understood by
both the child and their carers, is necessary. Holistic consid-
eration of the needs and capabilities of the family unit is
important in ensuring effective CKD care. Engage ment with
patients and families must change over the course of child-
hood from being entirely carer-directed for infants, changing
to include the whole family unit in child hood, and then
leaning toward the young person to ensure successful tran-
sition to adult-oriented care.
Child/adolescent
• Growth
• Nutrition
• Weight/BSA-based drug dosing
• Neurocognitive development
• Supporting education
• Transition to adult care
• Holistic approach to care for
the whole family unit
Pregnancy/lactation
• Drug pharmacokinetics
and pharmacodynamics
• Drug teratogenicity
• Risk of CKD progression
• Increased risk of pregnancy
complications, preterm birth
and small for gestational
age babies
• Fertility
Older adults
• Multidimensionality of
chronic conditions/
multimorbidity
• Frailty (including sarcopenia)
• Cognitive function
• Polypharmacy
• Prioritization
• End-of-life care
Gender
• Gender identity
• Gender roles
• Gender relations
• Institutionalized
gender
Sex
• Menopause
• Contraception
of risk factors and
complications
Figure 4 | Special considerations for chronic kidney disease (CKD) care across the lifespan. BSA, body surface area.
www.kidney-international.org special considerations
Kidney International (2024) 105 (Suppl 4S), S117–S314 S141

Growth, puberty, and young adulthood. Childhood and
adolescence are characterized by physical growth and devel-
opment. All CKD care aims to optimize these physiological
processes, which are commonly disrupted by CKD. Puberty is
a time of rapid somatic growth with an increase in muscle
bulk and therefore constitutes a high-risk period for CKD
progression as compromised kidneys may not hypertrophy to
adapt to the larger body size. Adolescence and emerging
adulthood bring individuation and exploration of sexuality
and adult behaviors, and kidney disease care must recognize
and adapt to these changes.
Kidney development and long-term assessment of kidney
risks.
Although nephron formation is complete by 36 weeks of
gestation, kidney function continues to develop throughout
early childhood, with nephron growth and maturatio n pro-
gressing particularly rapidly in the first year of life. An actual
increase in GFR over the course of the first 1–2 years of life, and
even up to 4 years of age, is expected. A trajectory of increasing
GFR in infancy and very early childhood followed by a period of
relative stability and a subsequent progression in CKD in
adolescence or adulthood is common. Given the long life ex-
pectancy of children, follow-up plans mu st take into account
the risk of late CKD or kidney failure. Healthy children and
adolescents should have excellent kidney function, so an esti-
mated eGFR under 90 ml/min per 1.73 m
2
(CKD G2–G5)
represents decreased kidney function in these age groups. Early
assessment and intervention of children with CKD is crucial to
maximize overall health across the lifespan.
Neurodevelopment and education. A primary goal of pedi-
atric CKD care is to optimize neurodevelopmental gains.
CKD can affect development, cognition, school attendance,
vocational outcomes, and future employment. Mitigating
these deficits through effective, individualized care is essential
to give children with CKD the best possible future.
Considerations in older adults
Older adults constitute a substantial and steadily growing
proportion of people under nephrology and medical care
globally, especially in Western industrialized countries.
Longevity in many parts of the world is increasing, and thus
the prevalence of CKD in those people is also increasing. The
2022 US Renal Data System (USRDS) annual data report
highlights that the number of individuals initiating KRT is
continuously ascending with increasing age. In Taiwan, for
example, KRT incidence in those aged 75þ was 2858 per
million population (pmp) compared with 1583 pmp among
people aged 65–74 years, 530 pmp among people aged 45 – 64
years, and 97 pmp among people aged 20–44 years. The
pattern is very similar across the g lobe with the majority of
people initiating dialysis over the age of 75, which puts
emphasis on a group of people who are not just old, but very
old, and incorporates more and more people over the age of
80. Octo- and nonagenarians often demonstrate distinct
patterns of disease complexity. These features include multi-
morbidity often accompanied by polypharmacy, frailty,
cognitive impairment, and gerontopsychiatric disorders
among others. Often, several of these features coexist espe-
cially in older adults with CKD.
Implications for agin g adult s with CKD are important in
both diagnosis and treatment. The interpretation of labora-
tory results (specifically SCr) used in the staging system
should factor in an older adult’s habitus given the frequency
of sarcopenia. A creatinine-based eGFR (eGFRcr) will over-
estimate GFR in the elderly (and others) with sarcopenia
leading to drug overdosing. Urine ACR at the same time will
be falsely high due to the falsely low creatinine in the de-
nominator. Furthermore, the presence of frailty may alter
treatment targets recommended for younger people with
CKD, as they may not necessarily be transferable to older
adults. Strict BP-lowering, for example, may come with the
risk of dizziness, falls, and fractures in older adults, many of
whom are on anticoagulants risking severe hemorrhage.
The multidimensionality of comorbidities in old age poses
challenges, as it demands a sophisticated integrated and
complex multidisciplinary care and treatment approach,
which may not be available in every healthcare system. Life
expectancy in old age is naturally limited compared with
younger people. Perspectives and treatment goals shift over
the life course, and recognizing these in very old adults, as
different from those in middle-aged or younger adults with
CKD, is critical to the development of more personalized care
plans and goals. Specifically, pure survival may become less of
a priority for an older individual, whereas maintaining an
acceptable, good quality of life (QoL) may be more impor-
tant. The context of a person’s situation and own values and
preferences may modify the prioritizati on for testing, treat-
ment types, and treatment goals. For example, the decision-
making between KRT and conservat ive care should be made
on the basis of the person’s priorities, medical needs, and
informed decision as to benefits and harms of various op-
tions. These informed decisions require good communication
between caregivers, people with CKD, and their relatives/
carers; they require time, “room,” adequate understandable
language, patience, trust, and commitment. Repeated con-
versations are critical, g iven the higher prevalence of cognitive
deficits in older adults with CKD. These cognitive issues
accompany both aging and CKD and frequently remain un-
recognized, thus, impeding shared decision-making and
advance care planning in this group.
In summary, older adults constitute the largest group
among all people with advanced CKD. Although every single
person needs individual care, the multidimensional medical
complexity inherent in very old age is challenging. Where
specific recommendations or practice points require special
consideration in the elderly, we make clear statements in the
special considerations section and encourage clinicians to
individualize therapies and goals of care in all patients, with
special attention to those of advanced age.
Considerations regarding sex and gender
It is increasingly recognized that sex (biological attributes)
and gender (sociocultural factors) factors across individuals
special considerations www.kidney-international.org
S142
Kidney International (2024) 105 (Suppl 4S), S117–S314

contribute to differences in kidney health and disease.
37–39
Sex-based variat ion in genetics, physiolog y, immunology, and
anatomy, as well as gender factors such as identity, roles, and
relations in addition to institutionalized gender, influences
kidney disease pathophysiology, presentation, response to
therapy, complications, and outcomes, highlighting the need
to take these factors into consideration in the care of the
person living with kidney disease.
Globally, the prevalence of CKD not being treated with
dialysis defined by level of eGFR is greater in women than
men.
40
Progression of CKD has been reported as more rapid
in men,
41,42
in women,
43
or no difference by sex or gender.
44
These incongruities are likely a reflection of diff erences in
cause of kidney disease and definitions of outcomes (e.g.,
loss of eGFR or receipt of KRT).
There is substantial literature demonstrating that both sex -
and gend er-related factors (e.g., puberty, menstrual patterns,
hormonal contraception, pregnancy and pregnancy-related
complications, menopause, menopausal hormone therapy,
testosterone levels, and gender-affirming hormone therapy)
play important roles in the risk, progression, complications,
and treatment of kidney disease.
45
These factors will play prominent roles in progression of
kidney disease across different stages of the life cycle. For
example, the use of some recommended medications has not
been studied in pregnant populations, highlighting the
importance of contraceptive counseling in accordance with a
person’s values and preferences. In other instances, precon-
ception counseling, changing medications to nonteratogenic
options and a multidisciplinary approach, is required to
optimize the outcomes of a potential pregnancy in the setting
of CKD. Sex-based differences in pharmacokinetics and
pharmacodynamics that are accentuated with increasing age
and changing hormonal status may alter the response to
different therapies for the treatment of kidney disease. For
example, women are more likely to report adverse reactions
to angiotensin-converting enzyme inhibitors (ACEi),
46
which
play a role in adherence and failure to reach guideline-
recommended target doses.
There are differences between women and men in the
detection, recognition, monitoring, referrals, and manage-
ment of CKD.
47,48
Although the reasons behind these
disparities are unclear, access to kidney care may be limited
by familial and other caregiving responsibilities, as well as
financial challenges, occupational obligations, and time
constraints, which are influenced by gender identity (how
an individual self-identifies, behaves, expresses their gender,
and i s perce ived by others, e.g., woman, man, girl, b oy,
and gender-diverse), roles (social expectations and norms
ty pically associated with a g iven gender, e.g., pr imar y
household earner and caregiver), relations (interactions
with and trea tment by ot hers based on an individual’s
perceived and/or expressed gender iden tity), and
institutionalized gender (e.g., distribution of power and
resources in soc iety).
37
A small but increasing proportion of the world’s popula-
tion identifies as transgender, gender-diverse, or nonbinary
where sex assigned at birth differs from gender identity,
highlighting the urgent need to build transgender cultural
safety within all aspects of kidney disease management and
care.
49
Taking sex and gender considerations into account is
critical to optimize the care of the individual with kidney
disease. Although there is increasing literature to inform sex-
and gender-specific recommendations in nephrology, signif-
icant knowledge gaps remain, underscoring the importance of
a person-centered approach in kidney care.
Considerations regarding fertility and pregnancy
Neither fertility nor pregnancy in people w ith CKD was part
of the scope of work for this guideline update, but there will
be special consideration relating to fertility and pregnancy
requiring specific reference in relevant sections of the
guideline.
Fertility. CKD is associated with decreased female and male
fertility.
50,51
Progressively impaired function of the
hypothalamic-pituitary-gonadal axis appears to play a key
role in the pathophysiology, although multiple factors
contribute to the reduction in fertility in this populati on. In
conjunction with the decreased fertility associated in CKD
and the uncertainty of the impact of assisted reproductive
technologies on kidney function, ongoing discussion of
family planning potential between the person with CKD
and their healthcare provider is essential.
Pregnancy. People with CKD are at risk for adverse preg-
nancy-associated outcomes, including progression of their
underlying CKD, a flare of their kidney disease, and adverse
pregnancy complications including pre-eclampsia, preterm
delivery, and small for gestational age infant.
52,53
The severity
of CKD is associated with risk of adverse pregnancy
outcomes. A multidisciplinary approach to preconception
counseling and man agement of pregnancy is necessary to
achieve optimal outcomes for both the person with CKD
and the infant.
54
www.kidney-international.org special considerations
Kidney International (2024) 105 (Suppl 4S), S117–S314 S143

Summary of relative and absolute risks relevant to CKD
from meta-analysis of large multinational population
studies in the CKD Prognosis Consortium (CKD-PC)
Outcomes relevant to CKD, and the prognostic importance of
CKD categories
The most highly evaluated endpoints in epidemiological
studies have been all-cause mortality, cardiovascular events
(myocardial infarction, stroke, and heart failure), and kidney-
specific outcomes (progression to kidney failure and AKI),
although additional outcomes such as all-cause hospitaliza-
tion and incident atrial fibrillation have been studied more
recently. In this section, we highlight newer data derived from
the CKD Prognosis Consor tium (CKD-PC).
12
We describe
the associations of CKD categories with 10 of these
important outcomes and demonstrate the importance of
different methods of estimating GFR (i.e., using creatinine-
or cystatin C–based equations) on these risk gradients.
Healthcare providers, researchers, and policy makers
should understand the association of CKD parameters (ACR
and eGFR) in populations. The overall distributions of
epidemiolog ical risk across CKD categories on a population
level are presented here. This is not to be confused with the
information presented in Chapter 2, where individualized risk
assessment tools are described, and those tools can be used to
inform clinical and management decisions for individual
people with CKD.
Associations of all complications o f CKD are incre-
mentally increased with worsened categories of estimated
glomerular filtration rate (eGFR) and albuminuria:
updated data.
The KDIGO 2012 Clinical Practice Guideline for the Evalu-
ation and Management of Chronic Kidney Disease introduced
the combined staging by eGFR and albuminuria categories,
which were justified by their associations with CKD complica-
tions.
1
The combined associations of eGFR and ACR categories
were presented as “heatmaps,” a color-coded depiction of the
associations of increased risk with worsening CKD, for
outcomes of all-cause mortality, kidney failure, AKI, and
cardiovascular mortality on a population level. In this section,
we provide an update to these CKD heatmaps, which have
been provided by the CKD-PC.
12
Several changes in the development of these updated
heatmaps are important to highlight.
(i) They now include several clinical databases that allow a
much larger population base, comprising up to 27,503,140
people for the analyses of each adverse outcome.
(ii) The eGFRcr has been changed to the 2021 CKD Epide-
miology Collaboration (CKD-EPI) equation, as this
newer version no longer includes race as a component.
(iii) The number of outcomes has been increased to 10,
including 6 that are cardiovascular related, 2 that are
kidney specific (kidney failure and AKI), and 2 general
outcomes (all-cause mortality and all-cause
hospitalization).
(iv) Additional analyses have been conducted using the 2021
CKD-EPI combined eGFR equation that incorporates
both creatinine and cystatin C. Although the sample size
for these subsequent analyses is much smaller (n ¼
720,736), it does permit better differentiation of associ-
ations of eGFR and risk and allows validation of CKD
thresholds across populations.
CKD staging by eGFRcr and ACR and association with adverse
events
Figure 5
12
presents the RRs for all eGFR/ACR combinations
for the 10 identified outcomes.
The RRs presented have all been adjusted for age, sex,
smoking status (current, former, or never), systolic BP (SBP),
total cholesterol, high-density lipoprotein (HDL) cholesterol,
body mass index (BMI), use of antihypertensive medications,
and a medical history of diabetes, coronary heart disease,
stroke, heart failure, atr ial fibrillation, peripheral artery dis-
ease, cancer, and chronic obstructive pulmonary disease.
Therefore, the RRs can be interpreted as the proportional
elevation in risk for each outcome experienced by people in
that stage of CKD (or non-CKD) compared with people in
the healthiest group. Across all the heatmaps, a consistent
color scheme is used.
The figures reveal severa l common themes a nd highlig ht
the necessity of having both eGFR and ACR parameters
available in assessing r isk. First, w ithin the CKD popula-
tion,theassociationofriskforall10outcomesincreases
with higher stages of both eGFR and albuminuria. The
fig u res pres e n t only th e RRs for ea c h spe ci ficstageandnot
the absolute risk of experiencing that outcome for people
in the risk cell. This distinction between relative and ab-
solute risks demonstrates the importance of using indi-
vidual risk prediction tools for persons w ith CKD, a subject
of Chapter 2.
Although nearly all CKD categories are at substantially
elevated risk for most outcomes in Figure 5, a distinction must be
made for people in the eGFRcr CKD G3a category and with the
lowest ACR severity (<10 mg/g [<1mg/mmol]).Thisgroupis
portrayed in the lower-risk green color for 7 of the 10 outcomes
presented, although they have 3-fold higher adjusted risk of AKI
and 13-fold higher risk of kidney failure compared with the
reference group. The inconsistent risk association for
populations with CKD G3a, A1, particularly in older adults,
has led to controversy over whether this group should be
relative and absolute risks associated with CKD www.kidney-international.org
S144
Kidney International (2024) 105 (Suppl 4S), S117–S314

considered as having CKD.
55
The CKD-PC investigators
repeated all 10 heatmaps using creatinine- and cystatin C–
based eGFR (eGFRcr-cys), in part to evaluate whether the
weaker associations of CKD G3a, A1 with clinical outcomes
were caused by the limitations of the specific creatinine-based
equation eGFRcr, compared with eGFRcr-cys, which has been
established as a better approximation of measured GFR
(mGFR) than eGFRcr (Figure 6
12
).
Overall
eGFRcr <10 10–29 30–299 300–999 1000+ <10 10–29 30–299
105+
1.6 2.2 2.9 4.3 5.8 1.1 1.4 2.0
90–104
ref 1.3 1.8 2.6 3.1 ref 1.3 1.6
60–89
1.0 1.3 1.7 2.2 2.8 1.1 1.3 1.6
45–59
1.3 1.6 2.0 2.4 3.1 1.4 1.7 2.0
30–44
1.8 2.0 2.5 3.2 3.9 1.9 2.0 2.4
15–29
2.8 2.8 3.3 4.1 5.6 2.7 3.1 3.1
<15
4.6 5.0 5.3 6.0 7.0 4.6 5.6 4.8
105+
1.4 2.0 3.0 4.1 5.4 1.2 1.6 2.2
90–104
ref 1.3 1.9 2.7 3.6 ref 1.3 1.6
60–89
1.0 1.4 1.7 2.4 3.2 1.1 1.3 1.7
45–59
1.4 1.7 2.2 2.8 3.8 1.4 1.6 1.9
30–44
2.0 2.3 2.8 3.7 4.6 1.6 1.7 2.0
15–29
3.2 3.1 3.5 5.0 6.5 1.8 2.1 2.1
<15
6.1 6.4 6.4 7.3 8.2 3.2 2.8 2.9
105+
0.5 1.2 2.9 7.7 25 1.2 1.7 2.7
90–104
ref 1.8 4.3 12 43 ref 1.3 2.0
60–89
2.3 4.9 10 27 85 1.1 1.4 1.9
45–59
13 19 37 89 236 1.6 1.8 2.4
30–44
50 58 115 240 463 2.2 2.5 3.1
15–29
283 301 443 796 1253 3.6 3.5 4.1
<15
770 1040 1618 2297 2547 5.1 5.7 5.8
105+
1.0 1.6 2.4 3.7 5.5 1.1 1.3 1.7
90–104
ref 1.4 2.1 3.2 5.0 ref 1.2 1.5
60–89
1.6 2.2 3.1 4.3 6.7 1.0 1.2 1.4
45–59
3.5 4.0 5.1 6.9 9.0 1.2 1.3 1.5
30–44
5.6 5.9 6.8 8.6 11 1.4 1.5 1.7
15–29
8.3 8.0 8.5 9.9 10 1.9 1.8 2.0
<15
8.5 11 7.9 5.5 5.7 2.6 2.5 3.1
105+
1.4 1.7 2.1 2.1 2.3 0.9 1.4 1.9
90–104
ref 1.1 1.3 1.5 1.7 ref 1.3 1.9
60–89
1.0 1.1 1.3 1.5 1.8 1.0 1.3 1.8
45–59
1.3 1.3 1.5 1.7 2.1 1.5 1.7 2.1
Urine albumin-creatinine r g/gm ,oitar eninitaerc-nimubla enirUg/gm ,oita
All-cause mortality: 82 cohorts
26 444 384
participants; 2 604 028 events
Myocardial infarction: 64 cohorts
22 838 356 participants; 451 063 events
Kidney failure with replacement therapy: 57 cohorts
25 466 956 participants; 158 846 events
Heart failure: 61 cohorts
24 603 016 participants; 1 132 443 events
Cardiovascular mortality: 76 cohorts
26 022 346 participants; 776 441 events
Stroke: 68 cohorts
24 746 436 participants; 461 785 events
Acute kidney injury: 49 cohorts
23 914 614 participants; 1 408 929 events
22 886 642 participants; 1 068 701 events
Hospitalization: 49 cohorts
25 426 722 participants; 8 398 637 events
Peripheral artery disease: 54 cohorts
24 830 794 participants; 378 924 events
30–44
1.5 1.5 1.6 1.9 2.3 2.0 1.9 2.5
15–29
1.8 1.8 1.9 2.4 2.8 3.3 3.3 3.8
<15
2.7 2.8 3.0 3.2 3.8 9.1 9.0 9.6
300–999 1000+
2.7 3.8
2.2 3.2
2.2 3.1
2.8 3.7
3.2 4.3
4.2 5.1
6.0 6.0
3.1 4.3
2.4 3.1
2.2 3.0
2.3 2.9
2.4 3.0
2.7 3.0
3.2 3.8
4.2 6.9
2.8 4.2
2.7 4.2
3.4 5.0
4.2 6.5
5.8 8.1
7.9 9.9
2.4 3.5
1.9 2.3
1.7 2.2
1.8 2.4
2.0 2.4
2.6 3.0
3.6 4.2
2.8 5.0
2.8 4.3
2.5 3.8
2.9 4.2
3.6 5.0
5.7 8.1
13 14
Figure 5 | Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by creatinine (eGFRcr) and
albumin-to-creatinine ratio (ACR) categories and risks for 10 common complications in multivariable-adjusted analyses. Numbers
reflect the adjusted hazard ratio compared with the reference cell. Adjustment variables included age, sex, smoking status (current, former, or
never), systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, use of antihypertensive medications,
and a medical history of diabetes, coronary heart disease, stroke, heart failure, atrial fibrillation, peripheral artery disease, cancer, and chronic
obstructive pulmonary disease, where relevant. The colors were determined for each outcome separately using the following rule: the
percentile shaded the darkest green color corresponds to the proportion of cells in the grid without CKD (e.g., 6 of 35 cells with eGFR $60 ml/
min per 1.73 m
2
and ACR <30 mg/g [<3 mg/mmol]), and the percentile shaded the darkest red color corresponds to proportion expected to be
at highest risk (e.g., 11 of 35 cells with eGFR <15 ml/min per 1.73 m
2
and albumin-to-creatinine ratio 1000þ mg/g [100þ mg/mmol]). In this
manner, the numbers of green and red cells are consistent across outcomes, but the patterns are allowed to differ. ref, reference cell.
Reproduced with permission from JAMA, Writing Group for the CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K, et al. Estimated
glomerular filtration rate, albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330(13):1266–1277.
12
Copyright ª 2023 American Medical Association. All rights reserved.
www.kidney-international.org relative and absolute risks associated with CKD
Kidney International (2024) 105 (Suppl 4S), S117–S314 S145

CKD staging by eGFRcr-cys and ACR and risk for adverse
events
Within the CKD-PC collaboration, 720,736 indiv iduals had
measures of blood cystatin C in addition to having eGFRcr
and ACR. The replacement of eGFRcr with eGFRcr-cys in the
heatmap led to several changes in the risk distributions. Most
notably, the groups with eGFR category 45–59 ml/min per
1.73 m
2
and ACR <10 mg/g [<1 mg/mmol] were moved to
eGFRcr-cys <10 10–29 30–299 300+ <10 10–29 30–299
105+
90–104
60–89
45–59
30–44
<30
105+
90–104
60–89
45–59
30–44
<30
105+
90–104
60–89
45–59
30–44
<30
105+
90–104
60–89
45–59
30–44
<30
105+
90–104
60–89
45–59
Urine albumin-creatinine ratio, mg/g Urine albumin-creatinine ratio, mg/g
All-cause mortality: 11 cohorts
692 802 participants; 97 006 events
Myocardial infarction: 10 cohorts
649 365 participants; 17 926 events
Kidney failure with replacement therapy: 5 cohorts
630 370 participants; 4306 events
Heart failure: 9 cohorts
641 298 participants; 27 406 events
Cardiovascular mortality: 11 cohorts
692 322 participants, 25 322 events
Stroke: 9 cohorts
662 605 participants; 16 909 events
Acute kidney injury: 5 cohorts
630 370 participants; 24 062 events
607 102 participants; 37 278 events
Hospitalization: 3 cohorts
630 489 participants; 464 894 events
Peripheral artery disease: 6 cohorts
642 624 participants; 3943 events
30–44
<30
300+
1.0 1.3 1.6 2.5 0.9 1.2 1.4 2.8
ref 1.3 1.5 2.0 ref 1.2 1.4 1.8
1.2 1.5 1.9 2.5 1.2 1.4 1.5 1.9
1.7 2.2 2.5 3.3 1.6 1.9 2.3 3.3
2.3
2.6
3.4 4.4 2.1 2.6 3.1 3.3
3.6 4.0 5.5 7.1 5.1 3.0 4.9 5.0
1.0 1.4 1.8 4.1 1.0 1.2 1.6 2.5
ref 1.5 1.6 2.9 ref 1.2 1.5 2.3
1.2 1.7 2.3 3.4 1.2 1.4 1.8 2.5
1.9 2.7 3.2 4.6 1.6 1.7 2.1 2.7
2.5 3.5 4.5 5.9 1.7 2.0 2.3 2.6
5.8 5.0 6.1 8.7 1.9 2.3 2.8 4.4
0.6 0.8 2.3 10 0.9 1.2 1.7 3.7
ref 1.5 4.5 11 ref 1.3 1.4 2.5
1.9 3.7 8.3 31 1.2 1.6 1.9 3.0
5.8 13 25 73 1.5 2.2 3.0 4.1
20 23 78 191 2.5 2.9 4.1 5.7
111 261 343 580 5.3 4.8 6.5 7.7
0.8
1.0
1.4 3.5 0.9 1.0 1.1 1.9
ref 1.3 1.7 2.8 ref 1.2 1.4 2.2
1.6 2.5 2.9 5.3 1.1 1.3 1.5 2.0
3.9 4.7 5.5 7.5 1.3 1.6 1.8 2.2
5.8 7.0 8.4 10 1.6 2.0 2.2 2.5
11 12 12 21 2.0 2.0 2.7 4.4
1.0 1.1 1.1 1.6 0.9 1.9 1.8 2.9
ref 1.1 1.3 1.4 ref 1.5 2.0 3.2
1.1 1.2 1.3 1.6 1.3 1.8 2.1 3.9
1.3 1.4 1.5 1.7 2.5 3.7 3.3 4.0
1.5 1.5 1.6 2.1 4.0 3.7 4.5 6.9
1.8 2.0 2.1 3.0 7.8 4.5 9.0 12
Figure 6 | Associations of chronic kidney disease (CKD) staging by estimated glomerular filtration rate by creatinine and cystatin C
(eGFRcr-cys) and albumin-to-creatinine ratio categories and risks for 10 common complications in multivariable-adjusted analyses.
Numbers reflect the adjusted hazard ratio compared with the reference cell. Adjustment variables included age, sex, smoking status (current,
former, or never), systolic blood pressure, total cholesterol, high-density lipoprotein cholesterol, body mass index, use of antihypertensive
medications, and a medical history of diabetes, coronary heart disease, stroke, heart failure, atrial fibrillation, peripheral artery disease, cancer,
and chronic obstructive pulmonary disease, where relevant. The colors were determined for each outcome separately using the following rule:
the percentile shaded the darkest green color corresponds to the proportion of cells in the grid without CKD (e.g., 6 of 24 cells), and the
percentile shaded the darkest red color corresponds to proportion expected to be at highest risk (e.g., 5 of 24 cells). In this manner, the numbers
of green and red cells are consistent across outcomes, but the patterns are allowed to differ. ref, reference cell. Reproduced with permission
from JAMA, Writing Group for the CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration rate,
albuminuria, and adverse outcomes: an individual-participant data meta-analysis. JAMA. 2023;330(13):1266–1277.
12
Copyright ª 2023 American
Medical Association. All rights reserved.
relative and absolute risks associated with CKD www.kidney-international.org
S146
Kidney International (2024) 105 (Suppl 4S), S117–S314

higher risk for all 10 outcomes, and this cell was no longer
labeled green for any of the complications (Figure 6). The
distinction in the se risk relationships was further explored
using spline analyses to depict the risk relationships of
eGFRcr and eGFRcr-cys with all the 10 complications. For
the 8 outcomes that are not influenced by changes in
creatinine (all except kidney failure and AKI), eGFRcr
exhibited a J-shaped association such that risk increased
with eGFR values over 105 ml/min per 1.73 m
2
(Figure 7
12
). In contrast, eGFRcr-cys demonstrated much
more linear associations with each of these complications
throughout its distribution.
Based upon the risk relationships of eGFRcr-cys and
ACR categories with all complications, the existing CKD
staging is appropriate among both younger and older
adults.
Some authors have suggested that the GFR threshold for
CKD of 60 ml/min per 1.73 m
2
should be raised to 75 ml/min
per 1.73 m
2
for younger adults and lowered to 45 ml/min per
1.73 m
2
for older adults.
55
In younger adults, the purpose of a
higher GFR threshold reflects the longer risk horizon for
younger people, which could lead to higher lifetime CKD
progression risks for a given GFR stage. However, the
higher lifetime progression risks in younger adults with
GFR 60–89 ml/min per 1.73 m
2
can be addressed in their
management without changing the definition of CKD.
Efforts should be directed at people with higher risk with
GFR levels >60 ml/min per 1.73 m
2
to prevent the
incidence of CKD or further reductions in GFR.
Among older adults, the findings of consistently elevated
RR for older adults with CKD G3a, A1, as defined by eGFRcr-
cys, support the inclusion of this large group in the CKD
population. These elevated RRs tell us how much more likely
the outcome is compared with the reference group (eGFR 90–
104 ml/min per 1.73 m
2
and ACR <10 mg/g [<1 mg/
mmol]). Crucially, they do not tell us what the overall like-
lihood of the outcome, the absolute risk, is. The absolute risk
for important CKD complications is higher among older than
younger adults at nearly every stage, particularly for CVD,
heart failure, and mortalit y. Therefore, this population is also
likely to benefit from having their CKD diagnosed, staged,
and treated.
Rationale for using cystatin C containing equations for CKD
staging
The rationale for using cystatin C versus SCr, or a combi-
nation of both, in eGFR equations is that creatinine, which is
directly linked to muscle mass, may be misleading at extremes
of body habitus, or in specific conditions (spinal cord injuries
and sarcopenia), and that cystatin C is impacted by different
variables (steroid use, thyroid disease, and cancer). Thus,
because neither is a perfect marker to use for estimating
0.7
1
1.5
2
3
4
Hazard ratio
0.7
1
1.5
2
3
4
Hazard ratio
15 30 45 60 75 90 105 120
eGFRcr, ml/min/1.73 m
2
eGFRcr-cys, ml/min/1.73 m
2
15 30 45 60 75 90 105 120
All-cause mortality, 721394 participants; 102 910 events
Cardiovascular mortality, 719987 participants; 27 051 events
All-cause hospitalization, 676 519 participants; 7862 events
Myocardial infarction, 711 478 participants; 18 659 events
Stroke, 711293 participants; 17 609 events
Heart failure, 674 255 participants; 28 530 events
Peripheral artery disease, 660 412 participants; 4458 events
Kidney failure with replacement therapy, 637 387 participants; 24 342 events
Acute kidney injury, 632 452 participants; 466 201 events
ba
Figure 7 | Hazard ratios for adverse outcomes using the continuous model of estimated glomerular filtration rate (eGFR), comparison
of the shape of associations between creatinine-based eGFR (eGFRcr) and creatinine and cystatin C–based eGFR (eGFRcr-cys) in the
population with cystatin C (eGFRcr-cys population). (a) Associations of eGFR based on creatinine alone with all-cause mortality,
cardiovascular mortality, all-cause hospitalizations, myocardial infarction, stroke, heart failure, atrial fibrillation, and peripheral artery disease; (b)
Associations of eGFR based on creatinine and cystatin C with all-cause mortality, cardiovascular mortality, all-cause hospitalizations, myocardial
infarction, stroke, heart failure, atrial fibrillation, and peripheral artery disease. Reproduced with permission from JAMA, Writing Group for the
CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an
individual-participant data meta-analysis. JAMA. 2023;330(13):1266–1277.
12
Copyright ª 2023 American Medical Association. All rights reserved.
www.kidney-international.org relative and absolute risks associated with CKD
Kidney International (2024) 105 (Suppl 4S), S117–S314 S147

clearance, the combination of the 2 compounds gives more
accurate estimates of GFR when compared with measured
values.
Very low levels of SCr often represent poor health status,
such as frailty or sarcopenia, which limits the production of
creatinine. This biological feature of creatinine (i.e., relation
to muscle mass) has limited its prognostic utility and results
in reducing the risk associations for eGFRcr 45– 60 ml/min
per 1.73 m
2
and elevating risks for eGFRcr >110 ml/min per
1.73 m
2
. These limitations are not observed when risk is
estimated using eGFRcr-cys or cystatin C–based eGFR
(eGFRcys) (Figure 7).
When comparing GFR estimates using these 2 filtration
markers, risk gradients are consistently stronger for most
outcomes for eGFRcys in comparison with eGFRcr. Therefore,
for the purpose of evaluating the association of eGFR with
outcomes (i.e., projecting prognosis for people with CKD), the
eGFRcys or eGFRcr-cys can be considered more accurate.
relative and absolute risks associated with CKD www.kidney-international.org
S148
Kidney International (2024) 105 (Suppl 4S), S117–S314

Summary of recommendation statements and practice
points
Chapter 1: Evaluation of CKD
1.1 Detection and evaluation of CKD
1.1.1 Detection of CKD
Practice Point 1.1.1.1: Test people at risk for and with chronic kidney disease (CKD) using both urine albumin mea-
surement and assessment of glomerular filtration rate (GFR).
Practice Point 1.1.1.2: Following incidental detection of elevated urinary albumin-to-creatinine ratio (ACR), hematuria,
or low estimated GFR (eGFR), repeat tests to confirm presence of CKD.
1.1.2 Methods for staging of CKD
Recommendation 1.1.2.1: In adults at risk for CKD, we recommend using creatinine-based estimated glomer-
ular filtration rate (eGFRcr). If cystatin C is available, the GFR category should be
estimated from the combination of creatinine and cystatin C (creatinine and cystatin
C–based estimated glomerular fi ltration rate [eGFRcr-cys]) (1B).
1.1.3 Evaluation of chronicity
Practice Point 1.1.3.1: Proof of chron icity (duration of a minimum of 3 months) can be established by:
(i) review of past measurements/estimations of GFR;
(ii) review of past measurements of albuminuria or proteinuria and urine microscopic
examinations;
(iii) imaging findings such as reduced kidney size and reduction in cortical thickness;
(iv) kidney pathological findings such as fibrosis and atrophy;
(v) medical history, especially condit ions known to cause or contribute to CKD;
(vi) repeat measurements within and beyond the 3-month point.
Practice Point 1.1.3.2: Do not assume chronicity based upon a single abnormal level for eGFR and ACR, as the finding
could be the result of a recent acute kidney injury (AKI) event or acute kidney disease (AKD).
Practice Point 1.1.3.3: Consider initiation of treatments for CKD at first presentation of decrea sed GFR or elevated ACR
if CKD is deemed likely due to presence o f other clinical indicators.
1.1.4 Evaluation of cause
Practice Point 1.1.4. 1: Establish the cause of CKD using clinical context, personal and family history, social and envi-
ronmental factors, medications, physical examination, laboratory measures, im aging, and genetic
and pathologic diagnosis (Figure 8).
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S149

Practice Point 1.1.4.2: Use tests to establish a cause based on resources available (Table 6
22,98-100
).
Recommendation 1.1.4.1: We suggest performing a kidney biopsy as an acceptable, safe, diagnostic test to
evaluate cause and guide treatme nt decisions when clinically appropriate (2D).
1.2 Evaluation of GFR
1.2.1 Other functions of kidneys besides GFR
Practice Point 1.2.1.1: Use the term “GFR” when referring to the specific kidney function of glomerular filtration. Use the
more general term “kidney function(s)” when dealing with the totality of functions of the kidney.
Physical
exam
Nephrotoxic
medications
Symptoms and signs
of urinary tract
abnormalities
Symptoms and signs
of systemic diseases
Laboratory tests, imaging, and tissue sample, such as:
• Urinalysis and urine sediment
• Urine albumin-to-creatinine ratio
• Serologic tests
• Ultrasound
• Kidney biopsy
• Genetic testing
Medical
history
Social and
environmental
history
Obtain careful family history
for possible genetic causes,
including family pedigree for CKD
Figure 8 | Evaluation of cause of chronic kidney disease (CKD).
Table 6 | Guidance for the selection of additional tests for evaluation of cause
Test category Examples Comment or key references
Imaging Ultrasound, intravenous urography, CT kidneys
ureters bladder, nuclear medicine studies, MRI
Assess kidney structure (i.e., kidney shape, size, symmetry, and evidence of
obstruction) for cystic disease and reflux disease.
Evolving role of additional technologies (e.g., 3D ultrasound)
Kidney biopsy Ultrasound-guided percutaneous Usually examined by light microscopy, immunofluorescence, and electron
microscopy, and, in some situations, may include molecular diagnostics
Used for exact diagnosis, planning treatment, assessing activity and
chronicity of disease, and likelihood of treatment response; may also be
used to assess genetic disease
Laboratory tests:
serologic, urine
tests
Chemistry including acid-base and electrolytes,
serologic tests such as anti-PLA2R, ANCA, anti-GBM
antibodies
Serum-free light chains, serum, and urine protein
electrophoresis/immunofixation
Urinalysis and urine sediment examination
Refer to KDIGO 2021 Clinical Practice Guideline for the Management of
Glomerular Diseases
22
Increasing recognition of the role of light chains in kidney disease even in
the absence of multiple myeloma (monoclonal gammopathy of renal
significance [MGRS])
98
Presence of persistent hematuria or albuminuria is critical in determining
differential diagnosis
Genetic testing APOL1, COL4A3, COL4A4, COL4A5, NPHS1, UMOD,
HNF1B, PKD1, PKD2
Evolving as a tool for diagnosis, increased utilization is expected.
Recognition that genetic causes are more common and may present
without classic family history
99,100
ANCA, antineutrophil cytoplasmic antibody; APOL1, apolipoprotein 1; COL4A, type IV collagen alpha chain; CT, computed tomography; GBM, glomerular basement membrane;
HNF1B, hepatocyte nuclear factor 1B; MRI, magnetic resonance imaging; NPHS1, congenital nephrotic syndrome; PKD1, polycystic kidney disease-1; PKD2, polycystic kidney
disease-2; PLA2R, M-type phospholipase A2 receptor; UMOD, uromodulin.
summary of recommendation statements and practice points www.kidney-international.org
S150
Kidney International (2024) 105 (Suppl 4S), S117–S314

1.2.2 Guidance to physicians and other healthcare providers
Practice Point 1.2.2.1: Use serum creatinine (SCr) and an estimating equation for initial assessment of GFR (Figure 11).
Recommendation 1.2.2.1: We recommend using eGFRcr-cys in clinical situations when eGFRcr is less accurate
and GFR affects clinical decision-making (Table 8
127-142
) (1C).
Practice Point 1.2.2.2: Where more accurate ascertainment of GFR will impact treatment decisions, measure GFR using
plasma or urinary clearance of an exogenous filtration marker (Table 9).
Practice Poi nt 1.2.2.3: Understand the value and limitations in both eGFR and measured glomerular filtration rate (mGFR)
as well as the variability and factors that influence SCr and cystatin C measurements.
Practice Point 1.2.2.4: Interpretation of SCr level requires consideration of dietary intake.
Initial test – eGFRcr*
Consider sources of error and need for
more accurate assessment.
Is eGFR thought to be accurate?
Consider potential sources of error in eGFRcr-cys
and need for an even more accurate assessment.
†
Is a more accurate assessment needed?
NoYes
No Yes
Measure
cystatin C
Evaluation of GFR for clinical application
15 30 45 60 90 120
Use eGFRcr
Measure
GFR
Use
GFRcr-cys
‡
Figure 11 | Approach to glomerular filtration rate (GFR) evaluation using initial and supportive tests. The algorithm describes the
approach to the evaluation of GFR. The approach uses initial and supportive testing to develop a final assessment of true GFR and to apply it in
individual decision-making. The initial test for the evaluation of GFR is creatinine-based estimated GFR (eGFRcr), which will be available for most
people because creatinine is measured routinely as part of the basic metabolic panel. If eGFRcr is expected to be inaccurate, or if a more
accurate assessment of GFR is needed for clinical decision-making, such as diagnosis or staging of chronic kidney disease or drug dosing, then,
if available, cystatin C should be measured, and creatinine and cystatin C–based estimated GFR (eGFRcr-cys) should be estimated. If eGFRcr-cys
is expected to be inaccurate, or if an even more accurate assessment of GFR is needed for clinical decision-making, then, if available, GFR should
be measured using plasma or urinary clearance of exogenous filtration markers. *Initial test may be estimated GFR by cystatin C (eGFRcys or
eGFRcr-cys) in otherwise healthy populations with changes in creatinine generation due to non-GFR determinants such as changes in muscle
mass or creatinine secretion or extrarenal elimination due to the use of specific medications.
†
Sources of error in eGFRcr-cys include very low
muscle mass or very high levels of inflammation, high catabolic states, or exogenous steroid use.
‡
Consider eGFRcys rather than eGFRcr-cys in
otherwise healthy populations with decreased creatinine generation due to reduced muscle mass or decreased creatinine secretion or
extrarenal elimination due to the use of specific medications.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S151

Table 8 | Indications for use of cystatin C
Domain Specific clinical condition Cause of decreased accuracy Comments on GFR evaluation
Body habitus and changes
in muscle mass
Eating disorders
127
Non-GFR determinants of SCr eGFRcys may be appropriate if no comorbid illness
other than reduction in muscle mass.
Extreme sport/exercise/
body builder
Non-GFR determinants of SCr eGFRcys may be appropriate if an increase in muscle
mass is the only abnormality.
Above-knee amputation
128
Non-GFR determinants of SCr eGFRcys may be appropriate in those without other
comorbid conditions. Suggest eGFRcr-cys in
those with comorbid illness.
Spinal cord injury with
paraplegia/paraparesis or
quadriplegia/quadriparesis
Non-GFR determinants of SCr eGFRcys may be appropriate in those without other
comorbid illness. Suggest eGFRcr-cys in those
with comorbid illness.
Class III obesity
a,b
Non-GFR determinants of SCr
and SCys
eGFRcr-cys demonstrated to be most accurate.
Lifestyle Smoking
129-131
Non-GFR determinants of SCys Minimal data, suggest eGFRcr if no changes to non-
GFR determinants of SCr or comorbid illness.
Diet Low-protein diet Non-GFR determinants of SCr
Minimal data, suggest eGFRcr may be appropriate if
no changes to non-GFR determinants of SCr or no
comorbid illness.
Keto diets Non-GFR determinants of SCr
Vegetarian Non-GFR determinants of SCr
High-protein diets
and creatine supplements
Non-GFR determinants of SCr
Illness other than CKD Malnutrition Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
eGFRcr-cys may be less accurate because of
coexistence of malnutrition and inflammation.
Suggest using mGFR for treatment decisions
based on the level of GFR.
Cancer
a,132-137
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
eGFRcr-cys demonstrated to be most accurate in
populations studied but likelihood of lesser
accuracy in more frail people or in cancers with
high cell turnover. Suggest using mGFR for
treatment decisions based on the level of GFR.
Heart failure
a,138,139
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Although limited data, eGFRcys appears less biased
but all have low accuracy. Suggest using eGFRcr-
cys or eGFRcys for routine GFR evaluation. Suggest
using mGFR for treatment decisions based on the
level of GFR.
Cirrhosis
a,79,140,141
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Although limited data, eGFRcys appears less biased
but all have low accuracy. Suggest using eGFRcr-
cys or eGFRcys for routine GFR evaluation. Suggest
using mGFR for treatment decisions based on the
level of GFR.
Catabolic consuming
diseases
c
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Minimal data but eGFRcr-cys may be inaccurate.
Suggest using eGFRcr-cys vs. eGFRcr for routine
GFR evaluation. Suggest using mGFR for treatment
decisions based on the level of GFR.
Muscle wasting diseases
142
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Minimal data. One study shows large bias for both
eGFRcr and eGFRcys. Suggest using eGFRcr-cys
for routine GFR evaluation. Suggest using mGFR
for treatment decisions based on the level of GFR.
Medication effects Steroids (anabolic, hormone) Non-GFR determinants of SCr.
Effect on SCys not known
Physiological effect on SCys unknown, suggest
eGFRcr-cys.
Decreases in tubular secretion Non-GFR determinants of SCr eGFRcys may be appropriate if medication affects only
creatinine and no comorbid illness. Suggest using
mGFR for treatment decisions based on the level
of GFR.
Broad spectrum antibiotics
that decrease extrarenal
elimination
Non-GFR determinants of SCr eGFRcys may be appropriate if medication affects only
creatinine and no comorbid illness. Suggest using
mGFR for treatment decisions based on the level
of GFR.
eGFR, estimated glomerular filtration rate; eGFRcr, creatinine-based estimated GFR; eGFRcr-cys, creatinine and cystatin C–based estimated GFR; GFR, glomerular filtration rate;
mGFR, measured glomerular filtration rate; SCr, serum creatinine; SCys, serum cystatin C.
a
Data summarized in Adingwupu et al.
149
b
Obesity class III varies by region but commonly body mass index >40 or >35 kg/m
2
.
c
Catabolic consuming disease may include tuberculosis, AIDS, hematologic malignancies, and severe skin diseases. There are no data with measured glomerular filtration rate
(mGFR) to evaluate this directly.
summary of recommendation statements and practice points www.kidney-international.org
S152
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 1.2.2.5: Assess the potential for error in eGFR when assessing a change in GFR over time.
Practice Point 1.2.2.6: Consider the use of cystatin C–based estimated glomerular filtration rate (eGFRcys) in some specific
circumstances.
Practice Point 1.2.2.7: Understand the implications of differences between eGFRcr and eGFRcys, as these may be infor-
mative, in both direction and magnitude of those differences.
Practice Point 1.2.2.8: Consider timed urine collections for measured creatini ne clearance if mGFR is not available and
eGFRcr-cys is thought to be inaccurate.
1.2.3 Guidance to clinical laboratories
Practice Point 1.2.3.1: Implement the laboratory standards of care outlined in Table 11 to ensure accuracy and reliability
when assessing GFR using creatinine and cystatin C.
Practice Point 1.2.3.2: Given available reso urces, clinical laboratories may consider the possibility of measurement of both
creatinine and cystatin either as an in-house test or as a referred test.
Special considerations
Pediatric considerations.
Practice Point 1.2.3.3: Laboratories measuring creatinine in infants or small children must ensure their quality control
process include the lowest end of the expected range of values for the group of interest.
Practice Point 1.2.3.4: Consider the consistent use of enzymatic creatinine assays in children, given the hig her relat ive
contribution of non-creatinine chromogens to measured creatinine in children when using the
Jaffe assay, and the high prevalence of icteri c and hemolyzed samples in the neonatal period.
Practice Point 1.2.3.5: An eGFRcr level <90 ml/min per 1.73 m
2
can be flagged as “low” in children and adolescents over the
age of 2 years.
Table 9 | Comparison of estimated GFR and measured GFR
Estimated GFR by SCr and/or cystatin C Measured GFR
Inexpensive and easy to implement More expensive, more time-consuming, and invasive
Widely available and may also be used
at point of care, easily repeatable
Only available in certain centers
Methods to measure that do not require urine collections are available (i.e., plasma clearance)
Most protocols require repeat blood samples potentially over a long duration
Microsampling tests by fingerpick enable point-of-care testing. Testing has been described,
but not routinely performed
Not sufficiently accurate and precise
for all clinical situations
Accurate for GFR in all situations and across the GFR range. Requires individualized protocols
Lags behind changes in GFR Able to identify early changes in GFR
Subject to non-GFR determinant confounding Less influenced by non-GFR determinants
GFR, glomerular filtration rate; SCr, serum creatinine.
Table 11 | Implementation standards to ensure accuracy and reliability of GFR assessments using creatinine and cystatin C
Report eGFR in addition to the serum concentrations of filtration markers using validated equations.
Report eGFR rounded to the nearest whole number and relative to a body surface area (BSA) of 1.73 m
2
in adults using the units ml/min per 1.73 m
2
.
Reported eGFR levels <60 ml/min per 1.73 m
2
should be flagged as being low.
When reporting levels of filtration markers, report:
(i) SCr concentration rounded to the nearest whole number when expressed as standard international units (
m
mol/l) and rounded to the nearest
100th of a whole number when expressed as conventional units (mg/dl);
(ii) serum cystatin C concentration rounded to the nearest 100th of a whole number when expressed as conventional units (mg/l).
Measure filtration markers using a specific, precise (coefficient of variation [CV] <2.3% for creatinine and <2.0% for cystatin C) assay with calibration
traceable to the international standard reference materials and desirable bias (<3.7% for creatinine and <3.2% for cystatin C) compared with reference
methodology (or appropriate international standard reference method group target in external quality assessment [EQA] for cystatin C).
Use an enzymatic method to assay creatinine, where possible.
Separate serum/plasma from red blood cells by centrifugation within 12 hours of venipuncture.
When cystatin C is measured, measure creatinine on the same sample to enable calculation of eGFRcr-cys.
eGFR, estimated glomerular filtration rate; eGFRcr-cys, estimated glomerular filtration rate based on creatinine and cystatin C; GFR, glomerular filtration rate; SCr, serum
creatinine.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S153

1.2.4 Selection of GFR estimating equations
Recommendation 1.2.4.1: We recommend using a validated GFR estimating equation to derive GFR from serum
filtration markers (eGFR) rather than relying on the serum filtration markers alone (1D).
Practice Point 1.2.4.1: Use the same equation within geographical regions (as defined locally [e.g., continent, country,
region] and as large as possible). Within such regions, equations may differ for adults and children.
Practice Point 1.2.4.2: Use of race in the computation of eGFR should be avoided.
Special considerations
Pediatric considerations.
Practice Point 1.2.4.3: Estimate GFR in children using validated equat ions that have been developed or validated in
comparable populations.
1.3 Evaluation of albuminuria
1.3.1 Guidance for physicians and other healthcare providers
Practice Point 1.3.1.1: Use the following measurements for initial testing of albuminuria (in descending order of pref-
erence). In all cases, a first void in the mo rning midstream sample is preferred in adults and
children.
(i) urine ACR, or
(ii) reagent strip urinalysis for albumin and ACR with automated reading.
If measuring urine protein, use the following measurements:
(i) urine protein-to-creatinine ratio (PCR),
(ii) reagent strip urinalysis for total protein with automated reading, or
(iii) reagent strip urinalysis for total protein with manual reading.
Practice Point 1.3.1.2: Use more accurate methods when albuminuria is detected using less accurate methods.
Confirm reagent strip positive albuminuria and/or proteinuria by quantitative laborat ory mea-
surement and express as a ratio to urine creatinine wherever possible (i.e., quantify the ACR or
PCR if initial semiquantitative tests are positive).
Confirm ACR ‡30 mg/g (‡3 mg/mmol) on a random untimed urine with a subsequent first
morning void in the morning midstream urine sample.
Practice Point 1.3.1.3: Understand factors that may affect interpretation of measurements of urine albumin and urine
creatinine and order confirmatory tests as indicated (Table 16).
Table 16 | Factors causing biological variation in urine albumin or urine protein
Factor Falsely elevated ACR or PCR False decrease in ACR or PCR
Variability in urine
albumin or protein
Hematuria Increases albumin and protein in the urine
Menstruation Increases albumin and protein in the urine
Exercise
259
Increases albumin and protein in the urine
Infection
260,261
Symptomatic urinary infection can cause
production of protein from the organism
Nonalbumin proteins Other proteins may be missed by albumin reagent strips
Variability in urinary
creatinine concentration
Biological sex Females have lower urinary creatinine excretion,
therefore higher ACR and PCR
Males have higher urinary creatinine excretion,
therefore lower ACR and PCR
Weight
73,160
Low urinary creatinine excretion consistent with low
weight can cause high ACR or PCR relative to
timed excretion
High urinary creatinine excretion consistent with high weight
can cause low ACR or PCR relative to timed excretion
Changes in creatinine
excretion
Lower urinary creatinine excretion with AKI
or low-protein intake
High urinary creatinine excretion with high-protein intake
or exercise
ACR, albumin-to-creatinine ratio; AKI, acute kidney injury; PCR, protein-to-creatinine ratio.
summary of recommendation statements and practice points www.kidney-international.org
S154
Kidney International (2024) 105 (Suppl 4S), S117–S314

Special considerations
Pediatric considerations.
Practice Point 1.3.1.4: In children, obtain a first morning urine sample for initial testing of albuminuria and proteinuria
(in descending order of preference):
(i) Both urine PCR and urine ACR,
(ii) Reagent strip urinalysis for total protein and for albumin with automated reading, or
(iii) Reagent strip urinalysis for total protein and for albumin with manual reading.
1.3.2 Guidance to clinical laboratories
Practice Point 1.3.2.1: Implement the laboratory reporting and handling standards outlined in Table 17 to ensure accuracy
and reliability of the findings when assessing urine samples.
Practice Point 1.3.2.2: Implementation of an external quality assessment scheme/program for urine albumin and creati-
nine, including calculation of the ACR, is a preferred practice for laboratories.
1.4 Point-of-care testing
Recommendation 1.4.1: We suggest that point-of-care testing (POCT) may be used for creatinine and urine
albumin measurement whe re access to a labora tory is limited or providing a test at the
point-of-care facilitates the clinical pathway (2C).
Practice Point 1.4.1: Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same pre-
analytical, analytical, and postanalytical quality criteria relating to the specimen collection and perfor-
mance of the device, including external quality assessment, and the interpretation of the result is used.
Practice P oint 1.4.2: Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the
equation consistent with that used within the region.
P ractice Point 1.4.3: Where a POCT device is being used for albuminuria testing, the capability of also analyzing
creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to
produce a positive result in 85% of people with significant albuminuria (ACR ‡30 mg/g or ‡3 mg/
mmol), as part of the evaluation and consideration of using the device.
Chapter 2: Risk assessment in people with CKD
2.1 Overview on monitoring for progression of CKD based upon GFR and ACR categories
Practice Point 2.1.1: Assess albuminuria in adults, or albuminuria/proteinuria in children, and GFR at least annually in
people with CKD.
Practice Point 2.1.2: Assess albuminuria and GFR more o ften for individuals at higher risk of CKD progression
when measurement will impact therapeutic decisions.
Practice Point 2.1.3: For people with CKD, a change in eGFR of >20% on a subsequent test exceeds the expected
variability a nd warrants evaluation.
Practice Point 2.1.4: Among people with CKD who initiate hemodyna mically active therapies, GFR reductions of
>30% on subsequent testing exceed the expected variability and warrant evaluation.
Practi ce Point 2.1.5: For albuminuria monitoring of people with CKD, a doubling of the ACR on a subsequent test
exceeds laboratory variability and warrants evaluation.
Table 17 | Implementation standards to ensure accuracy and reliability of urine samples
Samples for albumin measurement analyzed fresh or stored at 4
C for up to 7 days
Samples for albumin measurement should not be stored frozen at 20
C
Report ACR in untimed urine samples in addition to urine albumin concentration rather than the concentrations alone
Reporting to 1 decimal place for ACR whether mg/mmol or mg/g
Analytical CV of methods to measure urine albumin should be <15%.
ACR, albumin-to-creatinine ratio; CV, coefficient of variation.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S155

2.2 Risk prediction in people with CKD
Recommendation 2.2.1: In people with CKD G3–G5, we recommend using an externally validated risk equation
to estimate the absolute risk of kidney failure (1A).
Practi ce Point 2.2.1: A 5-year kidney failure risk of 3%–5% can be used to determine need for nephrology referral in
addition to criteria based on eGFR or urine ACR, and other clinical considerations.
Practice Point 2.2.2: A 2-year kidney failure risk of >10% can be used to determine the timing of multidisciplinary care
in addition to eGFR-based criteria and other clinical considerations.
Practi ce Poin t 2.2.3: A 2-year kidney failure risk threshold of >40% can be used to determine the modality education,
timing of preparation for kidney replacement therapy (KRT) including vascular access planning or
referral for transplantation, in addition to eGFR-based criteria and other clinical considerations.
Pra ctic e P o int 2. 2 . 4 : Note that risk prediction equations developed for use in people with CKD G3–G5, may not
be valid for use in those with CKD G1–G2.
Practi ce Point 2. 2.5: Use disease-specific, externally validated prediction equations in people with immunoglobulin
A nephropathy (IgAN) and autosomal dominant polycystic kidney disease (ADPKD).
2.3 Prediction of cardiovascular risk in people with CKD
Practice Point 2.3.1: For cardiovascular risk prediction to guide preventive therapies in people with CKD, use
externally validated models that a re either developed within CKD populations or that incor-
porate eGFR and albuminuria.
Prac ti ce Poi nt 2.3.2 : For mortality risk prediction to guide discussions about goals of care, use externally validated
models that predict all-cause mortality specifically developed in the CKD population.
Chapter 3: Delaying CKD progression and managing its complications
3.1 CKD treatment and risk modification
Practice Point 3.1.1: Treat people with CKD with a comprehensive treatment strategy to reduce risks of progression of
CKD and its associated complications (Figure 17).
3.2 Lifestyle factors
Practice Point 3.2.1: Encourage people with CKD to undertake physical activity compatible with cardiovascular
health, tol erance, a nd level of f railty; achieve an optimal body mass index (BMI); a nd not to use
tobacco products. Referral to provider s and programs (e.g., psycholo gists, rena l dietitians or
accredited nutrition providers, pharmacists, physical and occupational therapy, and smoking
cessation programs) should be offered where indicated and available.
3.2.1 Avoiding use of tobacco products
[No specific recommendations or practice points]
Impact on CKD pathophysiology
CKD manifestations
Prevention and treatment of clinical
symptoms and signs (including blood pressure)
Maximize health-related quality of life, physical function,
capacity to work, and ability to socialize
Appropriate monitoring and treatment of laboratory
abnormalities of CKD associated with implications for
health (e.g., anemia, CKD-MBD, potassium disorders, acidosis)
•
•
•
CKD outcomes
Minimize risk of progression to kidney failure
Manage risk and appropriate treatment of
complications, including cardiovascular diseases,
hospitalization, gout, infections, etc.
•
•
Modication of the natural course
of CKD and its symptoms
Figure 17 | Chronic kidney disease (CKD) treatment and risk modification. CKD-MBD, chronic kidney disease-mineral and bone disorders.
summary of recommendation statements and practice points www.kidney-international.org
S156
Kidney International (2024) 105 (Suppl 4S), S117–S314

3.2.2 Physical activity and optimum weight
The Work Group concurs with all the recommendation and practice points relating to physical activity from the KDIGO 2022
Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease
23
and considers that they should extend to all
adults with CKD. We draw attention to the following statements:
Recommendation 3.2.2.1: We recommend that people with CKD be advised to undertake moderate-intensity
physical activity for a cumulative duration of at least 150 minutes per week, or to a
level compatible with their cardiovascular and physical tolerance (1D).
Practice Point 3.2.2.1: Recommendations for physical activity should consider age, ethnic background, presence of other
comorbidities, and access to resources.
Practice Point 3.2.2.2: People with CKD should be advised to avoid sedentary behavior.
Practice Point 3.2.2.3: For people at higher risk of falls, healthcare providers should provide advice on the intensity of physical
activity (low, moderate, or vigorous) and the type of exercises (aerobic vs. resistance, or both).
Practice Point 3.2.2.4: Physicians should consider advising/encouraging people with obesity and CKD to lose weight.
Special considerations
Pediatric considerations.
Practice Point 3.2.2.5: Encourage children with CKD to undertake physical activity aiming for World Health Organization
(WHO)-advised levels (i.e., ‡60 minutes daily) and to achieve a healthy weight.
3.3 Diet
Practice Point 3.3.1: Advise people with CKD to adopt healthy and diverse diets with a higher consumption of plant-based
foods compared to animal-based foods and a lower consumption of ultraprocessed foods.
Practice Point 3.3.2: Use renal dietitians or accredited nutrition providers to educate people with CKD about dietary
adaptations regarding sodium, phosphorus, potassium, and protein intake, tailored to their indi-
vidual needs, and severity of CKD and other comorbid conditions.
3.3.1 Protein intake
Recommendation 3.3.1.1: We suggest maintaining a protein intake of 0.8 g/kg body weight/d in adults with
CKD G3–G5 (2C).
Practice Point 3.3.1.1: Avoid high protein intake (>1.3 g/kg body weight/d) in adults with CKD at risk of progression.
Practice Point 3.3.1.2: In adults with CKD who are willing and able, and who are at risk of kidney failure, consider
prescribing, under close supervision, a very low–protein diet (0.3–0.4 g/kg body weight/d) sup-
plemented with essential amino acids or ketoacid analogs (up to 0.6 g/kg body weight/d).
Practice Point 3.3.1.3: Do not prescribe low- or very low–protein diets in metabolically unstable people with CKD.
Special considerations
Pediatric considerations.
Practice Point 3.3.1.4: Do not restrict protein intake in children with CKD due to the risk of growth impairment. The
target protein and energy intake in children with CKD G2–G5 should be at the upper end of the
normal ran ge for healthy children to promote optimal growth.
Older adults.
Practice Point 3.3.1.5: In older adults with underlying conditions such as frailty and sarcopenia, consider higher protein
and calorie dietary targets.
3.3.2 Sodium intake
The Work Group concurs with the following recommendation from KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease
23
and the KDIGO 2021 Clinical Practice Guideline for the Management of Blood
Pressure in Chronic Kidney Disease.
21
Recommendation 3.3.2.1: We suggest that sodium intake be <2 g of sodium per day (or <90 mmol of sodium
per day, or <5 g of sodium chloride per day) in people with CKD (2C).
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S157

Practice Point 3.3.2.1: Dietary sodium restriction is usually not appropriate for patients with sodium-wasting nephropathy
Special considerations
Pediatric considerations.
Practice Point 3.3.2.2: Follow age-based Recommended Daily Intake when counseling about sodium intake for children with
CKD who have systolic and/or diastolic blood pressure >90th percentile for age, sex, and height.
3.4 Blood pressure control
The Work Group concurs with the KDIGO 2021 Clinical Practice Guideline fo r the Management of Blood Pressure in Chronic
Kidney Disease, which encourages individualized BP targets and the use of agents according to age, coexistent CVD, and other
comorbidities; risk of progression of CKD; and tolerance to treatments.
21
We highlight the following guidance:
Recommendation 3.4.1: We suggest that adults with high BP and CKD be treated with a target systolic blood pressure
(SBP) of <120 mm Hg, when tolerated, using standardized office BP measurement (2B).
Practice Point 3.4.1: Consider less intensive BP-lowering therapy in people with frailty, high risk of falls and fractures,
very limited life expectancy, or symptomatic postural hypotension.
Special considerations
Pediatric considerations.
The Work Group concurs with the KDIGO 2021 Clinical Practice Guideline fo r the Management of Blood Pressure in Chronic
Kidney Disease, and we highlight the following guidance
21
:
Recommendation 3.4.2: We suggest that in children with CKD, 24-hour mean arterial pressure (MAP) by
ambulatory blood pressure monitoring (ABPM) should be lowered to £50th percentile
for age, sex, and height (2C).
Practi ce Po i nt 3. 4.2 : Monitor BP once a year with ABPM and every 3 – 6 m onths with standardized ausc ultatory of fice
BP in children with CKD.
Practice Po int 3.4.3 : In children with CKD, when ABPM is not available, it is reasonable to target manual auscultatory
office SBP, obtained in a protocol-driven standardized setting, of 50th–75th percentile for age, sex, and
height unless achieving this target is limited by signs or symptoms of hypotension.
3.5 Glycemic control
Please refer to the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease for specific
recommendations, practice points, and research recommendations.
3.6 Renin-a ngiotensin system inhibitors
The Work Group highlights recommendations from the KDIGO 2021 Clinical Practice Guideline for the Management of Blood
Pressure in Chronic Kidney Disease and selected practice points for treatment with RASi from the KDIGO 2021 Clinical
Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease
21
and the KDIGO 2022 Clinical
Practice Guideline for Diabetes Management in Chronic Kidney Disease.
23
The Work Group considers several
recommendations to apply even in the absence of high BP and has adapted the recommendations from the BP guideline to
remove this requirement. Key recommendations and practice points are highlighted:
Recommendation 3.6.1: We recommend starting renin-angiotensin-system inhibitors (RASi) (an giotensin-con-
verting enzyme inhibitor [ACEi] or angiotensin II receptor blocker [ARB]) for people
with CKD and severely increased albumi nuria (G1–G4, A3) without diab etes (1B).
Recommendation 3.6.2: We suggest starting RASi (ACEi or ARB) for people with CKD and moderately increased
albuminuria (G1–G4, A2) without diabetes (2C).
Recommendation 3.6.3: We recommend starting RASi (ACEi or ARB) for people with CKD and moderately-to-
severely increased albuminuria (G1–G4, A2 and A3) with diabetes (1B).
Recommendation 3.6.4: We recommend avoiding any combination of ACEi, ARB, and direct renin inhibitor
(DRI) therapy in people with CKD, with or without diabete s (1B).
summary of recommendation statements and practice points www.kidney-international.org
S158
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 3.6.1: RASi (ACEi or ARB) should be administered using the highest approved dose that is tolerated to
achieve the benefits described because th e proven benefits were achieved in trials using these doses.
Practice Point 3.6.2: Changes in BP, serum creatinine, and serum potassium should be checked within 2–4 weeks of
initiation or increase in the dose of a RASi, depending on the current GFR and serum potassium.
Practice Point 3.6.3: Hyperkalemia associated with use of RASi can often be managed by measures to reduce the serum
potassium levels rather than decreasing the dose or stopping RASi.
Practice Point 3.6.4: Continue ACEi or ARB therapy unless serum creatinine rises by more than 30% within 4 weeks
following initiation of treatment or an increase in dose.
Practice Point 3.6.5: Consider reducing the dose or discontinuing ACEi or ARB in the setting of either symptomatic hy-
potension or uncontrolled hyperkalemia despite medical treatment, or to reduce uremic symptoms
while treating kidney failure (estimated glomerular filtration rate [eGFR] <15 ml/min per 1.73 m
2
).
Practice Point 3.6.6: Consider starting people with CKD with normal to mildly increased albuminuria (A1) on RASi (ACEi or
ARB) for specific indications (e.g., to treat hypertension or heart failure with low ejection fraction).
Practice Point 3.6 .7: Continue ACEi or ARB in people with CKD even when the eGFR falls below 30 ml/min per 1.73 m
2
.
3.7 Sodium-glucose cotransporter-2 inhibitors (SGLT2i)
The Work Group concurs w ith the KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney
Disease, which stated: “We recommend treating patients with type 2 diabetes (T2D), CKD, and an eGFR $20 ml/min per
1.73 m
2
with an SGLT2i (1A).”
23
However, in the present guideline, we offer a more general 1A recommendation for adults with
CKD. We also highlight practice points from the KDIGO Diabetes guideline for diabetes management in CKD, which are also
relevant for people with CKD without diabetes:
Recommendation 3.7.1: We recommend treating patients with type 2 diabetes (T2D), CKD, and an eGFR ‡20 ml/
min per 1.73 m
2
with an SGLT2i (1A).
Practice Point 3.7.1: Once an SGLT2i is initiated, it is reasonable to continue an SGLT2i even if the eGFR falls below
20 ml/min per 1.73 m
2
, unless it is not tolerated or KRT is initi ated.
Practice Point 3.7.2: It is reasonable to withhold SGLT2i during times of prolonged fasting, surgery, or critical medical
illness (when people may be at greater risk for ketosis).
Recommendation 3.7.2: We recommend treating adults with CKD with an SGLT2i for the following (1A):
eGFR ‡20 ml/min per 1.73 m
2
with urine ACR ‡200 mg/g (‡20 mg/mmol), or
heart failure, irrespectiv e of level of albuminuria.
Practice Point 3.7.3: SGLT2i initiation or use does not necessitate alteration of frequency of CKD monitoring and the
reversible decrease in eGFR on initiation is generally not an indication to discontinue therapy.
Recommendation 3.7.3: We suggest treating adults with eGFR 20 to 45 ml/min per 1.73 m
2
with urine ACR
<200 mg/g (<20 mg/mmol) with an SGLT2i (2B).
3.8 Mineralocorticoid receptor antagonists (MRA)
The Work Group highlights a key recommendation and practice points from the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease.
23
Recommendation 3.8.1: We suggest a nonsteroidal mineralocorticoid receptor antagonist with proven kidney
or cardiovascular benefit for adults with T2D, an eGFR >25 ml/min per 1.73 m
2
, normal
serum potassium concentration, and albuminuria (>30 mg/g [>3 mg/mmol]) despite
maximum tolerated dose of RAS inhibitor (RASi) (2A).
Practice Point 3.8.1: Nonsteroidal MRA are most appropriate for adults with T2D who are at high risk of CKD progression
and cardiovascular events, as demonstrated by persistent albuminuria despite other standard-of-care
therapies.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S159

Practice Point 3.8.2: A nonsteroidal MRA may be added to a RASi and an SGLT2i for treatment of T2D and CKD in adults.
Practice Point 3.8.3: To mitigate risk of hyperkalemia, select people with consistently normal serum potassium concen-
tration and monitor serum potassium regularly after initiation of a nonsteroidal MRA (Figure 26).
Practi ce Point 3.8.4: The choice of a nonsteroidal MRA should prioritize agents with documented kidney or
cardiovascular benefits.
Practice Point 3.8.5: A steroidal MRA may be used for treatment of heart failure, hyperaldosteronis m, or refractory
hypertension, but may cause hyperkalemia or a reversible decline in glomerular filt ration, particularly
among people with a low GFR.
3.9 Glucagon-like peptide-1 receptor agonists (GLP-1 RA)
The Work Group highlights a key recommendation and practice point from the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease.
23
Recommendation 3.9.1: In adults with T2D and CKD who have not achieved individualized glycemic targets
despite use of metformin and SGLT2 inhibitor treatment, or who are unable to use
those medications, we recommend a long-acting GLP-1 RA (1B).
Practice Point 3.9.1: The choice of GLP-1 RA should prioritize agents with documented cardiovascular benefits.
3.10 Metabolic acidosis
Practice Point 3.10.1: In people with CKD, consider use of pha rmacological treatment with or without dietary
intervention to prevent develo pment of acidosis with potential clinical implications (e.g., serum
bicarbonate <18 mmol/l in adults).
Practice Point 3.10.2: Monitor treatment for metabolic acidosi s to ensure it does not result in serum bicarbonate
concentrations exceeding the upper limit of normal and does not adversely affect BP control,
serum potassium, or fluid status.
3.11 Hyperkalemia in CKD
3.11.1 Awareness of factors impacting on potassium measurement
Practice Point 3.11.1.1: Be aware of the variability of potassium laboratory measurements as well as factors and mech-
anisms that may influence potassium measurement including diurnal and seasonal variation,
plasma versus serum samples, and the actions of medications.
3.11.2 Potassium exchange agents
Practice Point 3.11.2.1: Be aware of local availability or formulary restrictions with regard to the pharmacologic man-
agement of nonemergent hyperkalemia.
3.11.3 Timing to recheck potassium after identifying moderate and severe hyperkalemia in adults
[No recommendations and practice points]
K
+
≤4.8 mmol/l K
+
4.9–5.5 mmol/l
K
+
>5.5 mmol/l
• Initiate nerenone
- 10 mg daily if eGFR 25–59 ml/min/1.73 m
2
- 20 mg daily if eGFR ≥60 ml/min/1.73 m
2
• Monitor K
+
at 1 month after initiation and then every 4
months
• Increase dose to 20 mg daily, if on 10 mg daily
• Restart 10 mg daily if previously held for hyperkalemia and
K
+
now ≤5.0 mmol/l
• Continue nerenone 10 mg or 20 mg
• Monitor K
+
every 4 months
• Hold nerenone
• Consider adjustments to diet or concomitant
medications to mitigate hyperkalemia
• Recheck K
+
• Consider reinitiation if/when K
+
≤5.0 mmol/l
Figure 26 | Serum potassium monitoring during treatment with a nonsteroidal mineralocorticoid receptor antagonist (MRA)
(finerenone). Adapted from the protocols of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-
DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD). The Work Group considers
these potassium thresholds to be conservative, and it may be considered appropriate to continue MRAs in people with potassium of 5.5–6.0 mmol/
l. This algorithm could be used for steroidal MRA. The US Food and Drug Administration (FDA) has approved initiation of K
þ
< 5.0 mmol/l. This
figure is guided by trial design and the FDA label and may be different in other countries. Serum creatinine/estimated glomerular filtration rate
(eGFR) should be monitored concurrently with serum potassium. Reproduced from Kidney Disease: Improving Global Outcomes Diabetes Work
Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1–S127.
23
summary of recommendation statements and practice points www.kidney-international.org
S160
Kidney International (2024) 105 (Suppl 4S), S117–S314

3.11.4 Managing hyperkalemia
[No recommendations and practice points]
3.11.5 Dietary considerations
Practice Point 3.11.5.1: Implement an individualized approach in people with CKD G3–G5 and emergent hyperkalemia that
includes dietary and pharmacologic interventions and takes into consideration associated comor-
bidities and quality of life (QoL). Assessment and education through a renal dietitian or an accredited
nutrition provider are advised.
Practice Point 3.11.5.2: Provide advice to limit the intake of foods rich in bioavailable po tassium (e.g., processed foods)
for people with CKD G3–G5 who have a history of hyperkalemia or as a prevention strategy
during disease periods in which hyperkalemia risk may be a concern.
3.12 Anemia
The KDIGO 2012 Clinical Practice Guideline for Anemia in Chronic Kidney Disease will be updated in 2024.
437
3.13 CKD-Mineral Bone Disorder (CKD-MBD)
The Work Group highlig hts the KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and
Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD).
20
Please refer to this publication for specific
recommendations, selection, dosing of specific therapeutic agents, and research recommendations.
3.14 Hyperuricemia
Recommendation 3.14.1: We recommend people with CKD and symptomatic hyperuricemia should be offered
uric acid–lowering intervention (1C).
Practi ce Point 3.14.1: Consider initiating uric acid–lowering therapy for people with CKD after their first episode of gout
(particularly where there is no avoidable precipitant or serum uric acid concentration is >9 mg/dl
[535
m
mol/l]).
Practice Point 3.14.2 : Prescribe xanthine oxidase inhibitors in preference to uricosuric agents in people with CKD and
symptomatic hyperuricemia.
Practice Point 3.14.3: For symptomatic treatment of acute gout in CKD, low-dose colchicine or intra-articular/oral glu-
cocorticoids are preferable to nonsteroidal anti-inflammatory drugs (NSAIDs).
Dietary approaches.
Practice Point 3.14.4: Nonpharmacological interventions which may help prevent gout include limiting alcohol, meats, and
high-fructose corn syrup intake.
Recommendation 3.14.2: We suggest not using agents to lower serum uric acid in people with CKD and
asymptomatic hyperuricemia to delay CKD progression (2D).
3.15 Cardiovascular disease (CVD) and additional specific interventions to modify risk
3.15.1 Lip id management
The benefits of lowering LDL cholesterol using statin-based therapies on the risk of ASCVD are well established in people with
and without CKD. There are clear recommendations on when to initiate such therapies set out in the KDIGO Clinical Practice
Guideline for Lipid Management in Chronic Kidney Disease.
19
The Work Group concurs with all the recommendations in this
guideline. In particular, we draw attention to:
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S161

Recommendation 3.15.1.1: In adults aged ‡50 years with eGFR <60 ml/min per 1.73 m
2
but not treated with
chronic dialysis or kidney transplantation (GFR categories G3a–G5), we recom-
mend treatment with a statin or statin/ezetimibe combination (1A).
Recommendation 3.15.1.2: In adults aged ‡50 years with CKD and eGFR ‡60 ml/min per 1.73 m
2
(GFR cate-
gories G1– G2), we recommend treatment with a statin (1B).
Recommendation 3.15.1.3: In adults aged 18–49 years with CKD but not treated with chronic dialysis or kidney
transplantation, we suggest statin treatment in people with one or more of the
following (2A):
known coronary disease (myocardial infarction or coronary revascularization),
diabetes mellitus,
prior ischemic stroke, or
estimated 10-year incidence of coronary death or nonfatal myocardial infarction
>10%.
Practice Point 3.15.1.1: Estimate 10-year cardiovascular risk using a validated risk tool.
Practice Point 3.15.1.2: In people with CKD, choose statin-based regimens to maximize the absolute reduction in low-
density lipoprotein (LDL) cholesterol to achieve the largest treatment benefits.
Practice Point 3.15.1.3: In adults with CKD aged 18–49, a lower (i.e., <10%) estimated 10-year incidence of coronary death
or nonfatal myocardial infarction may also be appropriate thresholds for initiation of statin-based
therapy.
Practice Point 3.15.1.4: Consider prescribing proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors to people
with CKD who have an indication for their use.
Dietary approaches.
Practice Point 3.15.1.5: Consider a plant-based “Mediterranean-style” diet in addition to lipid-modifying therapy to reduce
cardiovascular risk.
3.15.2 Use of antiplatelet therapy
Recommendation 3.15.2.1: We recommend oral low-dose aspirin for prevention of recurrent ischemic cardio-
vascular disease events (i.e., secondary prevention) in people with CKD and
established ischemic cardiovascular disease (1C).
Practice Point 3.15.2.1: Consider other antiplatelet therapy (e.g., P2Y
12
inhibitors) when there is aspirin intolerance.
3.15.3 Invasive versus intensive medical therapy for coronary artery disease
Recommendation 3.15.3.1: We suggest that in stable stress-test confirmed ischemic heart disease, an initial
conservative approach using intensive medical therapy is an appropriate alterna-
tive to an initial invasive strategy (2D).
Practice Point 3.15.3.1: Initial management with an invasive strategy may still be preferable for people with CKD with
acute or unstable coronary disease, unacceptable levels of angina (e.g., patient dissatisfaction), left
ventricular systolic dysfunction attributable to ischemia, or left main disease.
summary of recommendation statements and practice points www.kidney-international.org
S162
Kidney International (2024) 105 (Suppl 4S), S117–S314

3.16 CKD and atrial fibrillation
Practice Point 3.16.1: Follow established strategies for the diagnosis and management of atrial fibrillation (Figure 40).
Recommendation 3.16.1: We recommend use of non–vitamin K antagonist oral anticoagulants (NOACs) in
preference to vitamin K antagonists (e.g., warfarin) for thromboprophylaxis in atrial
fibrillation in people with CKD G1–G4 (1C).
Practice Point 3.16.2: NOA C dose adjustment for GFR is required, with caution needed at CKD G4–G5.
Practice Point 3.16.3: Duration of NOAC discontinuation before elective procedures needs to consider procedural bleeding
risk, NOAC prescribed, and level of GFR (Figure 44).
Step 1
Diagnosis
• In people with CKD, use opportunistic pulse-based screening (e.g., taking at when measuring BP), followed by a
wearable device or Holter ECG testing
Step 2
Prophylaxis against
stroke and systemic
thromboembolism
(they are likely to have an increased CHA
2
DS
2
-VASc risk factor for stroke and are at high risk even with a score of 0–1)
managed (e.g., alcohol advice, use of a proton pump inhibitor)
Step 3
Rate/rhythm control
†
• Use medical therapy (e.g., beta blockade) to control ventricular rate to less than about 90 bpm at rest to decrease
symptoms and related complications
• For people with persistent symptoms despite adequate rate control, consider rhythm control with cardioversion,
antiarrhythmic therapy and/or catheter ablation
Figure 40 | Strategies for the diagnosis and management of atrial fibrillation. *Consider dose adjustments necessary in people with
chronic kidney disease (CKD).
†
The following has been recommended as a standard package for diagnostic evaluation of new atrial fibrillation:
(i) a 12-lead electrocardiogram (ECG) to establish the diagnosis, assess ventricular rate, and check for the presence of conduction defects,
ischemia, or structural heart disease; (ii) laboratory testing for thyroid and kidney function, serum electrolytes, and full blood count; and (iii)
transthoracic echocardiography to assess left ventricular size and function, left atrial size, for valvular disease, and right heart size and function.
BP, blood pressure; CHA
2
DS
2
-VASc, Congestive heart failure, Hypertension, Age $75 (doubled), Diabetes, Stroke (doubled), Vascular disease,
Age 65 to 74, and Sex category (female); HAS-BLED, Hypertension, Abnormal liver/kidney function, Stroke history, Bleeding history or
predisposition, Labile international normalized ratio (INR), Elderly, Drug/alcohol usage.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S163

Chapter 4: Medication management and drug stewardship in CKD
4.1 Medication choices and monitoring for safety
Practice Point 4.1.1: People with CKD may be more susceptible to the nephrotoxic effects of medications. When prescribing
such medications to people with CKD, always consider the benefits versus potential harms.
Practice Point 4.1.2: Monitor eGFR, electrolytes, and therapeutic medication levels, when indicated, in people with CKD
receiving medications with narrow therapeutic win dows, potential adverse effects, or nephrotoxicity,
both in outpatient practice and in hospital settings.
Practice Point 4.1.3: Review and limit the use of over-the-co unter medicines and dietary or herbal remedies that may be
harmful for people with CKD.
Medications and pregnancy.
Practice Point 4.1.4: When prescribing medications to people with CKD who are of child-bearing potential, always review
teratogenicity potential and provide regular reproductive and contraceptive counseling in accordance
with the values and preferences of the person with CKD.
4.2 Dose adjustments by level of GFR
Practice Point 4.2.1: Consider GFR when dosing medications cleared by the kidneys.
Practice Point 4.2.2: For most people and clinical settings, validated eGFR equations using SCr are appropriate for drug
dosing.
Practice Point 4.2.3: Where more accuracy is required for drug-related decision-making (e.g., dosing due to narrow ther-
apeutic or toxic range), drug toxicity, or clinical situations where eGFRcr estimates may be unreliable,
use of equations that combine both creatinine and cystatin C, or measured GFR may be indicated.
Apixaban–Edoxaban–RivaroxabanDabigatran
Low risk High risk
No important bleeding risk and/or adequate local hemostasis possible:
perform at trough level (i.e., ≥12 or 24 h after last intake)
Low risk High risk
CrCl ≥80 ml/min ≥24 h ≥48 h ≥24 h ≥48 h
CrCl 50–80 ml/min ≥36 h ≥72 h ≥24 h ≥48 h
CrCl 30–50 ml/min
a
≥48 h ≥96 h ≥24 h ≥48 h
CrCl 15–30 ml/min
a
No ocial indication No ocial indication ≥36 h ≥48 h
CrCl <15 ml/min No ocial indication for use
There is no need for bridging with LMWH/UFH
Figure 44 | Advice on when to discontinue non–vitamin K antagonist oral anticoagulants (NOACs) before procedures (low vs. high risk).
The bold values deviate from the common stopping rule of $24-hour low risk, $48-hour high risk. Low risk is defined as a low frequency of
bleeding and/or minor impact of a bleed. High risk is defined as a high frequency of bleeding and/or important clinical impact. Adapted from
Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non–vitamin-K
antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38:2137–2149.
724 a
Many of these
people may be on lower dose of dabigatran (110 mg twice per day [b.i.d.]) or apixaban (2.5 mg b.i.d.), or have to be on the lower dose of
rivaroxaban (15 mg QD) or edoxaban (30 mg QD). Dabigatran 110 mg b.i.d. has not been approved for use by the US Food and Drug
Administration. CrCl, creatinine clearance, LMWH, low-molecular-weight heparin; UFH, unfractionated heparin. Reproduced from Turakhia MP,
Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes
(KDIGO) Controversies Conference. Eur Heart J. 2018;39:2314–2325.
710
ª The Author(s) 2018. Published by Oxford University Press on behalf of
the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-
Commercial License (http://creativecommons.org/licenses/by-nc/4.0/).
summary of recommendation statements and practice points www.kidney-international.org
S164
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 4.2.4: In people with extremes of body weight, eGFR nonindexed for body surface area (BSA) may be
indicated, especially for medications with a narrow therapeutic range or requiring a minimum
concentration to be effective.
Practice Point 4.2.5: Consider and adapt drug dosing in people where GFR, non-GFR determinants of the filtration
markers, or volume of distribution are not in a steady state.
4.3 Polypharmacy and drug stewardship
Practice Point 4.3.1: Perform thorough medication review periodically and at transitions of care to assess adherence,
continued indication, and potential drug interactions because people with CKD often have complex
medication regimens and are seen by multiple specialists.
Practice Point 4.3.2: If medications are discontinued during an acute illness, communicate a clear plan of when to restart
the discontinued medications to the affected person and healthcare providers, and ensure docu-
mentation in the medical record.
Practi ce Poi nt 4.3.3: Consider planned discontinuation of medications (such as metformin, ACEi, ARBs, and SGLT2i) in
the 48–72 hours prior to elective surgery or during the acute management of adverse effects as a
precautionary measure to prevent complications. However, note that failure to restart these medi-
cations after the event or procedure may lead to unintentional harm (see Practice Point 4.3.2).
4.3.1 Strategies to promote drug stewardship
Practi ce Poin t 4.3.1.1: Educate and inform people with CKD regarding the expected benefits and possible risks of medi-
cations so that they can identify and report adverse events that can be managed.
Practice Point 4.3.1.2: Establish collaborative relationships with other healthcar e providers and pharmacists and/or
use tools to ensure and improve drug stewardship in people with CKD to enhance manageme nt
of their complex medicati on reg imens.
4.4 Imaging studies
Practice Point 4.4.1: Consider the indication for imaging studies in accordance with general population in-
dications. Risks and benefits of imaging stud ies should be determined on an ind ividual
basis in the context of their CKD.
4.4.1 Radi ocontrast: intra-arterial and intravenous dye studies
Practice Point 4.4.1.1: Assess the risk for AKI in people with CKD receiving intra-arterial contrast for cardiac pro-
cedures using validated tools.
Practice Point 4.4.1.2: The intravenous administration of radiocontrast media can be managed in accordance with
consensus statements from the radiol ogy societies in people with AKI or GFR <60 ml/min per 1.73
m
2
(CKD G3a–G5) undergoing elective investigation.
4.4.2 Gadolinium-containing contrast media
Practice Point 4.4.2.1: For people with GFR <30 ml/min per 1.73 m
2
(CKD G4–G5) who require gadolinium-containing
contrast media, preferentially offer them A merican College of Radiology group II and III
gadolinium-based contrast agents.
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S165

Chapter 5: Optimal models of care
5.1 Referral to specialist kidney care services
Practice Point 5.1.1: Refer adults with CKD to specialist kidney care services in the circu mstances listed in Figure 48.
Special considerations
Pediatric considerations.
Practice Point 5.1.2: Refer children and adolescents to specialist kidney care services in the following circumstances:
an ACR of 30 mg/g (3 mg/mmol) or a PCR of 200 mg/g (20 mg/mmol) or more, confirmed on a
repeat first morning void sample, when well and not during menstruation,
persistent hematuria,
any sustained decrease in eGFR,
hypertension,
kidney outflow obstruction or anomalies of the kidney and urinary tract,
known or suspected CKD, or
recurrent urinary tract infection.
5.2 Symptoms in CKD
5.2.1 Prevalence and severity of symptoms
[No recommendations and practice points]
5.2.2 Identification and assessment of symptoms
Practice Point 5.2.2.1: Ask people with progressive CKD about uremic symp toms (e.g ., reduced appetite, nausea, and level
of fatigue/lethargy) at each consultation using a standardized validated assessment of uremic
symptoms tool.
Further evaluation and
specialist management
based on diagnosis
Causes
Circumstances category Circumstance examples Actions
Diagnosis of CKD
eGFR/risk of KRT
Planning and preparation
for kidney replacement
therapy
Albuminuria
and microscopic
hematuria
Further evaluation
and management
Others
Management of CKD
complications
• Cause of CKD is uncertain
• Hereditary kidney disease
• Recurrent extensive nephrolithiasis
• A >3%–5% 5-year risk of requiring KRT measured
using a validated risk equation
• eGFR <30 ml/min per 1.73 m
2
• A sustained fall in GFR of >20% or >30% in those
people initiating hemodynamically active therapies
• Consistent nding of signicant albuminuria
(ACR ≥300 mg/g [≥30 mg/mmol] or AER ≥300 mg/24 hours,
approximately equivalent to PCR ≥500 mg/g [≥50 mg/mmol]
or PER ≥500 mg/24 h) in combination with hematuria
• ≥2-fold increase in albuminuria in people with signicant
albuminuria undergoing monitoring
• A consistent nding of ACR >700 mg/g [>70 mg/mmol]
• Urinary red cell casts, RBC >20 per high power eld
sustained and not readily explained
• CKD and hypertension refractory to treatment
≥4 antihypertensive agents
• Persistent abnormalities of serum potassium
• Acidosis
• Anemia
• Bone disease
• Malnutrition
Figure 48 | Circumstances for referral to specialist kidney care services and goals of the referral. ACR, albumin-to-creatinine ratio; AER,
albumin excretion rate; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; PCR, protein-
creatinine ratio; PER, protein excretion rate; RBC, red blood cells.
summary of recommendation statements and practice points www.kidney-international.org
S166
Kidney International (2024) 105 (Suppl 4S), S117–S314

5.2.3 Management of common symptoms for people with CKD
Practice Point 5.2. 3.1: Use evidence-informed management strategies to support people to live well with CKD and improve
their health-related quality of life.
Practice Point 5.2.3.2: Screen people with CKD G4–G5, aged >65, poor growth (pediatrics), or symptoms such as
involuntary weight loss, frailty, or poor appetite twice annually for malnutrition using a
validated assessm ent to ol.
Practi ce Poin t 5.2.3.3: Enable availability of appropriate medical nutrition therapy for people with signs of malnutrition,
ideally under the supervision of renal dietitians or accredited nutrition providers if not available.
5.3 Team-b ased integrated care
Practice Point 5.3.1: Enable access to a patient-centered multidisciplinary care team consisting of dietary counseling,
medication management, education, and counseling about different KRT modalities, transplant
options, dialysis access surgery, and ethical, psychological, and social care for people with CKD.
Practice Point 5.3.2: Education programs that also involve care partners where indicated are important to promote
informed, activated people with CKD.
Practice Point 5.3.3: Consider the use of telehealth techno logies including web-based, mobile applications, virtual
visiting, and wearable devices in the delivery of education and care.
Special considerations
Pediatric considerations.
5.3.1 Transition from pediatric to adult care
5.3.1.1 Pediatric providers
Practice Point 5.3.1.1.1: Prepare adolescents and their families for transfer to adult-oriented care starting at 11–14 years of age
by using checklists to assess readiness and guide preparation, and by conducting part of each visit
without the parent/guardian present (Figure 55).
Practice Point 5.3.1.1.2: Provide a comprehensive written transfer summary, and ideally an oral handover, to the receiving
healthcare providers including all relevant medical information as well as informa tion about the
young person’s cognitive abilities and social support (Figure 55).
Practice Point 5.3.1.1.3: Transfer young people to adult care during t imes of medical and social stability where poss ible.
5.3.1.2 Adult providers
Practice Point 5.3.1.2.1: Recognize that young people under 25 years of age with CKD are a unique population at high risk for
adverse outcomes at least in part due to physiologic incomplete brain maturation.
Practice Point 5.3.1.2.2: Encourage young people to informally visit the adult care clinic to which they will be transferred
before the first appointment (Figure 55).
Transition
Preparation for transfer
Pediatric care
Preparation for regular adult care
Joint pediatric-adult care
or
Young adult care
Transition
Regular
adult care
Transfer – when stable
• Allow young people to visit the clinic before transfer
• Recognize that “emerging adulthood” is a period of
high risk for adverse outcomes
• See emerging adults more frequently than older adults
with same stage of CKD
• Include caregivers or signicant others in patient visits,
with permission of patient
Age (years)
11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
• Start early (11–14 yr)
• Use checklists to assess readiness
and guide preparation
• See young person alone for
at least part of each visit
• Comprehensive written
summary and verbal
handover, including
cognitive ability and
social support
• Follow-up after transfer
Figure 55 | The process of transition from pediatric to adult care in chronic kidney disease (CKD).
www.kidney-international.org summary of recommendation statements and practice points
Kidney International (2024) 105 (Suppl 4S), S117–S314 S167

Practice Point 5.3.1.2.3: Assess young people with CKD more frequently than older people with the same stage of CKD and,
with the agreement of the young person, include the caregivers or significant other of the young
person in their care, at least in the first 1–3 years following transfer from pediatric care (Figure 55).
5.4 Timing the initiation of dialysis
Practice Point 5.4.1: Initiate dialysis based on a composite assessment of a person’s symptoms, signs, QoL, preferences,
level of GFR, and laboratory abnormalities.
Practice Point 5.4.2: Initiate dialysis if the presence of one or more of the following situations is e vident ( Table 41).
This often but not invariably occurs in the GFR ran ge between 5 and 10 ml/mi n per 1.73 m
2
.
Practice Point 5.4.3: Consider planning for preemptive kidney transplantation and/or dialysis access in adults when the
GFR is <15–20 ml/min per 1.73 m
2
or risk of KRT is >40% over 2 years.
Special considerations
Pediatric considerations.
Practice Point 5.4.4: In children, in addition to the adult indications for dialysis, poor growth refractory to optimized
nutrition, growth hormone, and medical management is an indication for initiating KRT.
Practice Point 5.4.5: Pursue living or deceased donor preemptive kidney transplantation as the treatment of choice for
children in whom there is evidence of progressive and irreversible CKD. The eGFR at which pre-
emptive transplantation should be undertaken will depend on multiple factors including the age and
size of the child and the rate of progression of kidney failure but will usually be between 5–15 ml/min
per 1.73 m
2
.
5.5 Structure and process of supportive care and comprehensive conservative management
Practice Point 5.5.1: Inform people with CKD about the options for KRT and comprehensive conservative care.
Practice Point 5.5.2: Support comprehensive conservative management as an option for people who choose not to pursue
KRT.
Practice Point 5.5.3: Provide access to resources that enable the delivery of advanced care planning for people with a
recognized need for end-of-life care, including those people undergoing comprehensive conservative
care.
Table 41 | Indications for the initiation of dialysis
Symptoms or signs attributable to kidney failure (e.g., neurological signs and symptoms attributable to uremia, pericarditis, anorexia, medically resistant
acid-based or electrolyte abnormalities, intractable pruritus, serositis, and acid-base or electrolyte abnormalities)
Inability to control volume status or blood pressure
Progressive deterioration in nutritional status refractory to dietary intervention, or cognitive impairment
summary of recommendation statements and practice points www.kidney-international.org
S168
Kidney International (2024) 105 (Suppl 4S), S117–S314

Chapter 1: Evaluation of CKD
1.1 Detection and evaluation of CKD
Both decreased GFR and increased albuminuria or other
markers of kidney damage are often silent and not appare nt to
thepersonatriskofCKDorthehealthcareproviderunless
laboratory tests are performed. The cause of the decreased GFR
or increased albuminuria may also not be apparent. In the
decade since the publication of the previous KDIGO Clinical
Practice Guideline for the Evaluation and Management of
Chronic Kidney Disease,
1
there have been substantial advances
in treatment for CKD of all causes (Chapter 3), targeted
therapies for specific causes of CKD (e.g., KDIGO 2021
Clinical Practic e Guideline for the Management of
Glomerular Diseases
22
), as well as understanding of and
methods to determine the etiology of CKD. All together , these
advances have the potential to slow and possibly prev ent the
progression of kidney disease. Thus, in this section of Chapter
1, we emphasize the importance of detecting CKD, and
considerations for the optimal methods for staging of CKD,
and how to establish chronicity and etiology.
1.1.1 Detection of CKD
Practice Point 1.1.1.1: Test people at risk for and with chronic
kidney disease (CKD) using both urine albumin measurement
and assessment of glomerular filtration rate (GFR).
Early detection of any chronic disease, including CKD,
provides greater opportunities to reduce morbidity as treat-
ments can be initiated earlier in the disease course. Because
treatments for CKD provide benefits in reducing risk for both
CVD and CKD progression, strategies that promote early
detection of CKD should improve kidney and non–kidney-
related outcomes. Even if medical treatments are not available
or indicated for an individual, there are recommended lifestyle
changes that could be implemented after the diagnosis of CKD
(Chapter 3). Interviews with people who have CKD have pro-
vided evidence that many would alter their lifestyle if they
received a diagnosis of CKD.
17
Knowledge of level of
albuminuria and GFR also helps guide clinical decisions
beyond initiating treatments specifically for CKD (Table 4).
Each of these is considered in greater depth in the subsequent
chapters. Finally, if a familial form of kidney disease is
suspected, the diagnosis of the disease in one person may
allow detection in other family members. Thus, in itial testing
of blood and urine to detect CKD is important, with
confirmatory testing if initial findings indicate the presence of
abnormalities of creatinine/eGFR or albuminuria.
From a societal perspective, early identification of and
intervention for CKD could have a positive impact on health
disparities. In many countries, there is a higher incidence of CKD
among people with lower SES, and these people are more likely
to progress to kidney failure and have less access to KRT (dialysis
Table 4 | Use of GFR and albuminuria
Clinical decisions
Current level
Change in the level of GFRGFR Albuminuria
Diagnosis and
staging
Detection of CKD
Evaluation for kidney donation
Detection of CKD Detection of AKI and AKD
Detection of CKD progression
Treatment
Referral to nephrologists
Patient and family education about CKD
and benefit of lifestyle changes
Monitor progression of GFR decline
Referral for kidney transplantation
Placement of dialysis access
Dosage and monitoring for medications
cleared by the kidney
Determine safety of diagnostic tests or procedures
Eligibility for clinical trials
Referral to nephrologists
Patient and family education about
CKD and benefit of lifestyle changes
Monitor progression of GFR decline
Eligibility for clinical trials
Treatment of AKI
Monitoring drug toxicity
Re-evaluate CKD treatment
strategies
Risk assessment
Risk of CKD complications
Risk for CKD progression
Risk of CVD
Risk for medication errors
Risk for perioperative complications
Risk for mortality
Fertility and risk of complications
of pregnancy
Risk for CKD progression
Risk for CVD
Risk for mortality
Fertility and risk of complications
of pregnancy
Risk for kidney failure
Risk for CVD, HF, and mortality
Risk for adverse pregnancy
outcome
AKD, acute kidney disease; AKI, acute kidney injury; CKD, chronic kidney disease; CVD, cardiovascular disease; GFR, glomerular filtration rate; HF, heart failure.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S169

and transplantation).
56
A public health approach toward CKD
detection and treatment could reduce inequities in the burden
of kidney failure by slowing the rate of progression and the
risk of CVD for everyone.
57
The possible harm of early detection of CKD is that the
new diagnosis may cause anxiety in some people, particularly
if the testing is not discussed in advance of the results. Dis-
cussions around disease detection are common in the primary
care setting, and shared decision-making is an established
practice through which people may agree to the testing,
confirm that they would like to be tested, and prepare for the
range of possible results and their implications.
58–60
Early
detection increases burden and costs associated with physi-
cian visits or treatments, and this may not be balanced by
savings from averting adverse outcomes.
CKD fits the World Health Organization (WHO) criteria for
an early detection program.
61–63
Given that chronic disease
detection and prevention frameworks have been deployed for
other disease and risk factor conditions, in our view, CKD
detection strategies should be implemented for high-risk people.
A framework has been developed for communities to align
CKD detection and treatment strategies within their broader
public health priorities to ensure that the goals of the interven-
tion are achieved without compromising other valuable health
initiatives.
26
Both the efficacy and the cost-effectiveness of CKD
detection and treatment interventions will depend upon the
specific strategies that are employed in the healthcare system.
Therefore, results from future clinical trials should be
evaluated within their unique context and may not generalize
to all CKD detection efforts.
Most people with or at risk for CKD, healthcare providers, and
policy makers would wish to identify CKD. Most people who are
already receiving medical care would choose case-finding stra-
tegies to enable earlier risk stratification and treatment for pre-
viously undiagnosed CKD.
17,64
Thus, the application of earlier
treatment to delay CKD progression in people with CKD is of
a higher priority than the lack of clinical trial evidence that
case-finding strategies themselves improve outcomes.
This practice point promoting CKD detection efforts may
have implications for health equity, because CKD dispro-
portionately affects people from minoritized populations and
those who have lower SES. The increasing availability and
evidence supporting several treatments for CKD advocates for
early disease detection. Given the asymptomatic progression
of CKD, systematic testing of people with risk factors for
CKD is the only method that would detect CKD at early
stages and allow the initiation of appropriate treatments.
CKD detection could reduce the proportion of people with
CKD who will experience the morbidity of CKD G4–G5.
Cost-effectiveness analyses, performed in the new era of
effective disease-modifying therapies, describe a more positive
view of population-wide screening.
27
Figure 3
28
provides an algorithm for the identification of
people at risk for CKD testing in those at risk, further
testing in those identified as havi ng CKD to confirm stages,
and subsequently allowing for treatment initiation. Primary
care physicians or other medical specialists who care for
people with risk factors for CKD, such as endocrinology,
cardiology, or rheumatology, are ideal settings for an
intervention that targets people with undetected CKD.
Implementing an early detection intervention would be
facilitated by integrated healthcare systems and the use of
electronic health records. These structures would facilitate
the linkage between risk stratification and treatment to have
the desired effect of slowing the progression of CKD.
The highest priority conditions for CKD detection are
hypertension, diabetes, and CVD, including heart failure. For
diabetes, the ADA and KDIGO recommend annual screening
of people with diabetes for CKD.
29
CKD screening should
start at diagnosis of T2D because evidence of CKD is often
already apparent at this time. For T1D, screening is
recommended commencing 5 years after diagnosis. A
second important group includes people with recent AKI or
acute kidney disease (AKD), particularly multiple episodes
of AKI, and those who have been “partially diagnosed” with
CKD by either eGFR or albuminuria but cannot be fully
staged. Other groups who might be considered for CKD
testing are shown in Table 5. This list is not exhaustive and
may be modified by local epidemiological considerations. As
per above, 2023 analyses suggest that population screening
may in fact be cost-effective, obviating the need for
“selecting” and addressing an ever-changing list of “at risk”
groups.
27
Testing for CKD without regard to age generates contro-
versy. Those in older age groups experience the greatest
burden of CKD and are also at the highest risk for cardio-
vascular complications. As with other detection programs like
cancer detection, CKD detection efforts should be individu-
alized based on the person’s goals of care and suitability for
treatment.
Practice Point 1.1.1.2: Following incidental detection of
elevated urinary albumin-to-creatinine ratio (ACR), he-
maturia, or low estimated GFR (eGFR), repeat tests to
confirm pre sence of CKD.
There is known biological and analytical variability in SCr
and in urine albumin or urine protein not related to their
properties as markers of kidney disease. In people without
risk factors for CKD, there is a low pretest probability for
CKD. Thus, any unexpected results should be verified before
diagnosing a person as having CKD. In people with risk
factors for CKD, there is a higher probability that the person
does have CKD even with an unexpected finding. Subsequent
testing should be performed to confirm the diagnosis and to
complete the evaluation, as is required. Timing of the repeat
sample should be determined based on clinical setting
including risk factors for CKD as well as concern for AKI/
AKD.
Hematuria is common and associated with risk for sub-
sequent development of CKD.
65
There are several causes of
chapter 1 www.kidney-international.org
S170
Kidney International (2024) 105 (Suppl 4S), S117–S314

transient hematuria. Persistent hematuria may indicate
glomerular disease, other kidney diseases, or urologic
disease including genitourinary malig nancy. Thus, persistent
hematuria should prompt further investigation.
66,67
Special considerations
Pediatric considerations.
People who are born preterm,
especially if also small for gestational age, are at increased risk
for CKD and kidney failure. This is largely related to
decreased nephron number.
68–70
Additional insults after birth
such as neonatal AKI and childhood obesity can further in-
crease the risk of CKD.
71,72
1.1.2 Methods for staging of CKD
Recommendation 1.1.2.1: In adults at risk for CKD,
we recom mend using creatinine-based estimated
glomerular filtration rate (eGFRcr). If cystatin C is
available, the GFR category should be estimated
from the combination of creatinine and cystatin C
(creatinine and cystatin C–based estimated glomer-
ular filtration rate [eGFRcr-cys]) (1B) .
For the diagnosis and staging of CKD by GFR, this recom-
mendation puts a high value on data suggesting that the most
accurate method of estimating GFR is by using 2 biomarkers
(cystatin C and creatinine), as each has limitations and benefits
as filtration markers. As compared with mGFR, estimating
equations using both creatinine and cystatin C afford greater
accuracy in compa rison with either filtration marker alone. The
recommendation places a lower value on the resource utilization
and cost associated with the assessment of eGFRcr-cys.
Key information
Balance of benefits and harms.
In the CKD-PC collabora-
tion, 720,736 people had measures of blood cystatin C in addi-
tion to having eGFRcr and ACR.
12
Replacing the assessment of
eGFRcr with eGFRcr-cys in the matrix of GFR categories led
to several changes in the risk distributions. Most notably, the
group with an eGFR category 45–59 ml/min per 1.73 m
2
and
ACR <10 mg/g (<1 mg/mmol) was moved to higher risk for
all 10 outcomes, and this category was no longer labeled
as being low-risk (“green”) for any of the complications
(Figure 6
12
). For the 8 outcomes that are not influenced by
changes in creatinine (i.e. all except kidney failure and AKI),
eGFRcr exhibited a J-shaped association such that risk
increased with eGFR values >105 ml/min per 1.73 m
2
(Figure 7
12
). In contrast eGFRcr-cys demonstrated much
more linear associations with each of these complications
throughout its distribution. These data demonstrate that the
combined eGFRcr-cys equation is superior for distinguishing
GFR risk stages compared with eGFRcr.
Certainty of evidence. This recommendation is based on 2
broadly different types of data—data comparing the accuracy
(P
30
) of equations from a combination of creatinine and
cystatin C as filtration markers and creatinine and cystatin C
Table 5 | Risk factors for CKD
Domains Example conditions
Common risk factors Hypertension
Diabetes
Cardiovascular disease (including heart failure)
Prior AKI/AKD
People who live in geographical areas with
high prevalence of CKD
Areas with endemic CKDu
Areas with the high prevalence of APOL1 genetic variants
Environmental exposures
Genitourinary disorders Structural urinary tract disease
Recurrent kidney calculi
Multisystem diseases/chronic inflammatory
conditions
Systemic lupus erythematosus
Vasculitis
HIV
Iatrogenic (related to drug treatments and procedures) Drug-induced nephrotoxicity and radiation nephritis
Family history or known genetic variant
associated with CKD
Kidney failure, regardless of identified cause
Kidney disease recognized to be associated with genetic abnormality (e.g., PKD,
APOL1-mediated kidney disease, and Alport syndrome)
Gestational conditions Preterm birth
Small gestational size
Pre-eclampsia/eclampsia
Occupational exposures that promote CKD risk Cadmium, lead, and mercury exposure
Polycyclic hydrocarbons
Pesticides
AKD, acute kidney disease; AKI, acute kidney injury; APOL1, apolipoprotein L1; CKD, chronic kidney disease; CKDu, chronic kidney disease of undetermined origin; PKD,
polycystic kidney disease.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S171

alone and data from the CKD-PC examining the risk of
outcome by GFR stage assessed by eGFRcr compared with
eGFRcr-cys. As compared with equations based on creatinine
and cystatin C alone, the equation using both creatinine and
cystatin C comes closest to mGFR most consistently
(Supplementary Table S3
73–96
). The CKD-PC data were an
individual-level data analysis of 27,503,140 participants
from 114 global cohorts (eGFRcr) and 720,736 participants
from 20 cohorts (eGFRcr-cys) and 9,067,753 participants
from 114 cohorts (albuminuria) from 1980 to 2021 from
around the world conveying a high degree of robustness in
the association of CKD stage with a broad range of adverse
outcomes. Based on the totality and consistenc y of the
CKD-PC data, the overall certainty of the evidence was
rated as moderate.
Values and preferences. This recommendation places a high
value on the need for the most accurate assessment of GFR.
The Work Group judged that many people at risk for CKD
would prefer an accurate measurement when confirming the
diagnosis of CKD and its staging. For this reason, the Work
Group prioritized eGFRcr-cys over eGFRcr or eGFRcys for
the most accurate measurement. The recommendation puts a
low value on the availability and cost of an assessment of
eGFRcr-cys suggesting that people at risk of CKD would opt
for the more accurate assessment.
Resource use and costs. The costs and resource use associ-
ated with eGFRcr-cys are currently greater than those of
eGFRcr; however, the need for an accurate measurement may
offset these expenses. In addition, accurate diagnosis of CKD
as early as possible may lead to lower resource utilization and
healthcare spending than if diagnosed in later stages of CKD.
For more information on the costs assoc iated with cystatin C
assessments, please refer to Section 1.2.2.
Considerations for implementation. The biggest consideration
for implementation is the availability of cystatin C measurement.
For this reason, the recommendation includes the alternative for
eGFRcr in such cases taking into consideration the limitations
and drawbacks of creatinine-based measurements.
Rationale
The KDIGO CKD staging system based on 2 dimensions,
GFR and albuminu ria, was created largely to reflect the as-
sociation of outcomes of people with CKD, relative to the
earlier staging systems based solely on GFR stages. Assessment
of GFR stage is ideally performed using accurate assessment
of GFR and ACR and is used to best capture the prognosis for
people with CKD with regard to outcomes such as kidney
failure, CVD, and mortality risk. There is now a large evi-
dence base demonstrating that the use of eGFRcr-cys reclas-
sifies a large proportion of the population into different GFR
stages and the “new” stages better reflect their risk associa-
tions. For that reason, where available, cystatin C should be
added to creatinine for the purpose of estimating GFR for
CKD diagnosis and staging.
1.1.3 Evaluation of chronicity
Practice Point 1.1.3.1: Proof of chronicity (duration of a
minimum of 3 months) can be established by:
(i) review of past measurements/estimations of GFR;
(ii) review of past measurements of albuminuria or
proteinuria and urine microscopic examinations;
(iii) imaging findings such as reduced kidney size and
reduction in cortical thickness;
(iv) kidney pathological findings such as fibrosis and
atrophy;
(v) medical history, especially conditions known to
cause or contribute to CKD;
(vi) repeat measurements within and beyond the 3-
month po int.
Practice Point 1.1.3.2: Do not assume chronicity based
upon a single abnormal level for eGFR and ACR, as the
finding could be the result of a recent acute kidney injury
(AKI) event or acute kidney disease (AKD).
Practice Point 1.1.3.3: Consider initiation of treatments for
CKD at first presentation of decreased GFR or elevated
ACR if CKD is deemed likely due to presence of other
clinical indicators.
Kidney diseases may be acute or chronic.
1,97
We explicitly
yet arbitrarily define the duration of a minimum of 3 months
(>90 days) as delineating “chronic” kidney disease. The
rationale for defining chronicity is to differentiate CKD
from AKDs (such as acute glomerulonephritis [GN]),
including AKI, which may require different timelines for
initiation of treatments, different interventions, and have
different etiologies and outcomes. The duration of kidney
disease may be documented or inferred based on the
clinical context. For example, a person with decreased GFR
or kidney damage during an acute illness, without prior
documentation of kidney disease, may be inferred to have
AKD. Resolution over days to weeks would confirm the
diagnosis of AKI from a variety of different causes. A
person with similar findings in the absence of an acute
illness may be inferred to have CKD, and if followed over
time, would be confirmed to have CKD. In both cases,
repeat ascertainment of GFR and kidney damage is
recommended for accurate diagnosis and staging. The
timing of the evaluation depends on clinical judgment, with
earlier evaluation for those suspected of having AKI and
later evaluation for those suspected of having CKD.
For people w ith risk factors for CKD, delaying diag-
nosis for the sake o f confirm ing chronicit y can delay
care. Many people may not recognize the impor tance of
a repeat v isit if treatment had not been initiated. Thus,
initiating treatment both allows for earlier inter vention
and also indicates to people the importance of the
disease.
chapter 1 www.kidney-international.org
S172
Kidney International (2024) 105 (Suppl 4S), S117–S314

Special considerations
Pediatric considerations.
Newborns who clearly have kidney
disease (e.g., severe congenital malformations of the kidney and
urinary tract) do not need to wait 3 months to confirm CKD.
1.1.4 Evaluation of cause
Practice Point 1.1.4.1: Establish the cause of CKD using
clinical context, personal and family history, social and
environmental factors, medications, physical examination,
laboratory measures, imaging, and genetic and pathologic
diagnosis (Figure 8).
Practice Point 1.1.4.2: Use tests to establish a cau se based
on resources available (Table 6
22,98-100
).
In evaluation of cause, healthcare providers should select
specific diagnostic tests based on the pretest probability of a
specific diagnosis informed by clinical presentation. Identifica-
tion of cause confers benefit for targeting therapy to slow pro-
gression to kidney failure, understanding contributing factors,
and prognosis. In addition, identification of cause can help
people communicate information about a genetic or familial
cause to relatives, improve understanding of their condition in
the context of self-care management, and improve health literacy.
Genetic testing is emerging as a valuab le component for
evaluation of cause. In some studies, >10% of people with
CKD, regardless of family history, were observed to carr y
genetic pathogenic and likely pathogenic variant(s) that
represent a plausible molecular cause for the development or
progression of CKD.
101–103
In some cases, identification of
actionable genes through genetic testing can impact the
clinical management of people with CKD (Figure 9
100
).
104,105
The prevalence of genetic causes to CKD is expected to
increase in future years through increased recognition.
A recent KDIGO Controversies Conference listed the following
recommendations for when genetic testing can be particularly
informative: (i) high prevalence of monogenic subtypes within the
clinical category , (ii) early age of onset of CKD , (iii) syndromic/
multisystem features, (iv) consanguinity , (v) possibility of iden-
tifying a condition amenable to targeted treatment, and (vi) CKD/
kidney failure of unknown etiology when kidney biopsy would not
be informative due to advanced disease.
100
The KDIGO Controversies Conference also highlighted
the importance of an educated workforce with expertise in
kidney genetics, genomics, and computational research for
appropriate use and interpretation of these tests (Figure
10
100
). Access to genetic counseling and medical genetics is
important for psychosocial support, appropr iate use of
genetic testing, and to limit costs.
102
Most people with a new diagnosis of CKD and their
healthcare providers would prefer to undertake evaluation for
the underlying cause to ensure that the best possible care is
provided. Althoug h some people identified as having CKD
may prefer not to undergo the (sometimes invasive) pro-
cedures to evaluate cause, establishing cause enables the most
appropriate management strategy to be implemented.
Resources available for evaluation of cause will vary
worldwide. People may not be able to pay for some diagnostic
tests. Therefore, healthcare providers should tailor the eval-
uation of cause based on these resource constraints (e.g.,
urine protein reagent strip testing instead of ACR).
Education on the value of establishing a diagnosis of CKD
is critical. This can be done through local, national, and in-
ternational kidney societies and within healthcare training
programs (Chapter 5). Additional resources may be required
to support wider scale implementation of diagnostic tests,
especially genetic testing, availability of biopsies, and the
support required for implementation.
Identification of cause is often achieved by standard clin-
ical methods (i.e., history and examinat ion), knowledge of the
causes of CKD and their manifestations (i.e., level of GFR and
specific marker of kidney damage such as hematuria, urine
albumin, or cysts), together with specialized investigations
(Figure 8). Not all evaluations of cause are required in all
people. Information from the clinical context and initial
tests may lead to further evaluatio ns ( Table 6), which are
likely to be conducted as part of specialized kidney care
services and dependent on resources (Chapter 5).
Recommendation 1.1.4.1: We suggest performing a
kidney biopsy as an acceptable, safe, diagnostic test
to evalu ate cause and guide treatment decisions
when clinically appropriate (2D).
This recommendation places a high value on an acceptable safety
profile of kidney biopsies when used to evaluate the cause of
CKD and to plan appropriate treatment.
Key information
Balance of bene fits and harms.
The benefits of kidney bi-
opsy in terms of diagnosis, prognosis, and planning appro-
priate treatment for both the person with CKD and healthcare
Physical
exam
Nephrotoxic
medications
Symptoms and signs
of urinary tract
abnormalities
Symptoms and signs
of systemic diseases
Laboratory tests, imaging, and tissue sample, such as:
• Urinalysis and urine sediment
• Urine albumin-to-creatinine ratio
• Serologic tests
• Ultrasound
• Kidney biopsy
• Genetic testing
Medical
history
Social and
environmental
history
Obtain careful family history
for possible genetic causes,
including family pedigree for CKD
Figure 8 | Evaluation of cause of chronic kidney disease (CKD).
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S173

providers are through improved understanding of the iden-
tified disease state and the extent of active and chronic lesions.
The harms include the possibility of complications of the
procedure (bleeding risk/pain), the obtaining of a non-
diagnostic or insufficient sample (wasted resource), and the
anxiety induced while awaiting the results.
The systematic review performed by the ERT identified 37
studies assessing the prognostic benefi t and safety of kidney
biopsy among people with CKD. Ten studies examined the
diagnostic and/or prognostic benefit of kidney biopsy or in-
fluence of biopsy results on management decisions. The
diagnostic findings were heterogeneous and variable, which
did not lend themselves to further synthesis. The rate of
mortality after native kidney biopsy in people with suspected
or diagnosed CKD was low. Across the 15 studies that re-
ported on mortality after a native kidney biopsy, there were 3
reported deaths. The rate of perirenal hematoma across 14
studies was estimated to be 16% (95% confidence interval
[CI]: 12%–22%). No studies reported on retroperitoneal
hemorrhage (Supplementary Table S4
106–126
).
Certainty of evidence. The overall certainty of evidence for
kidney biopsy andoutcomes of harms is very low (Supplementary
Table S4
106–126
). The critical outcomes, mortality and perirenal
hematomas, were primarily assessed in observational studies
without a comparison group. Because of the potential for
confounding, the ERT cons ider ed the body of evidence to have
Table 6 | Guidance for the selection of additional tests for evaluation of cause
Test category Examples Comment or key references
Imaging Ultrasound, intravenous urography, CT kidneys
ureters bladder, nuclear medicine studies, MRI
Assess kidney structure (i.e., kidney shape, size, symmetry, and evidence of
obstruction) for cystic disease and reflux disease.
Evolving role of additional technologies (e.g., 3D ultrasound)
Kidney biopsy Ultrasound-guided percutaneous Usually examined by light microscopy, immunofluorescence, and electron
microscopy, and, in some situations, may include molecular diagnostics
Used for exact diagnosis, planning treatment, assessing activity and
chronicity of disease, and likelihood of treatment response; may also be
used to assess genetic disease
Laboratory tests:
serologic, urine
tests
Chemistry including acid-base and electrolytes,
serologic tests such as anti-PLA2R, ANCA, anti-GBM
antibodies
Serum-free light chains, serum, and urine protein
electrophoresis/immunofixation
Urinalysis and urine sediment examination
Refer to KDIGO 2021 Clinical Practice Guideline for the Management of
Glomerular Diseases
22
Increasing recognition of the role of light chains in kidney disease even in
the absence of multiple myeloma (monoclonal gammopathy of renal
significance [MGRS])
98
Presence of persistent hematuria or albuminuria is critical in determining
differential diagnosis
Genetic testing APOL1, COL4A3, COL4A4, COL4A5, NPHS1, UMOD,
HNF1B, PKD1, PKD2
Evolving as a tool for diagnosis, increased utilization is expected.
Recognition that genetic causes are more common and may present
without classic family history
99,100
ANCA, antineutrophil cytoplasmic antibody; APOL1, apolipoprotein 1; COL4A, type IV collagen alpha chain; CT, computed tomography; GBM, glomerular basement membrane;
HNF1B, hepatocyte nuclear factor 1B; MRI, magnetic resonance imaging; NPHS1, congenital nephrotic syndrome; PKD1, polycystic kidney disease-1; PKD2, polycystic kidney
disease-2; PLA2R, M-type phospholipase A2 receptor; UMOD, uromodulin.
Conditions amenable
to specific disease-
modifying therapies
Avoidance of
prolonged
immunosuppressive
therapies
Conditions at risk for
recurrence after
kidney transplantation
Conditions for which
genetic testing is
relevant for reproductive
counseling
Examples:
• GLA (Fabry)
• AGXT (primary
hyperoxaluria (PH))
• CoQ10 genes (SRNS)
• CTNS (cystinosis)
• Tubulopathies
(Na
+
, K
+
etc.)
Conditions amenable
to nonspecific
renoprotective
strategies
Example:
• COL4A3/4/5 (Alport)
and RAAS blockade
Example:
• Glomerular disease
due to mutations in
Alport genes
(COL4A3/4/5)
Examples:
• (CFH/CFI/C3...): aHUS
• (AGXT, GRHPR,
HOGA): primary
hyperoxaluria (PH)
• Adenine phosphoribo-
syltransferase deficiency
(APRT)
Conditions amenable
to specific screening
for extrarenal
manifestations
Examples:
• HNF1B: diabetes
• PKD1/PKD2
(ADPKD): intracranial
aneurysms
• FLCN: renal cell
carcinoma, etc.
Example:
• Prenatal/preimplantation
diagnosis
Figure 9 | Actionable genes in kidney disease. Actionability refers to the potential for genetic rest results to lead to specific clinical actions
from prevention or treatment of a condition, supported by recommendations based on evidence. ADPKD, autosomal dominant polycystic
kidney disease; aHUS, atypical hemolytic uremic syndrome, CKD, chronic kidney disease; RAAS, renin-angiotensin-aldosterone system, SRNS,
steroid-resistant nephrotic syndrome. Reproduced from KDIGO Conference Participants. Genetics in chronic kidney disease: conclusions from a
Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2022;101:1126–1141.
100
Copyright ª 2022, Kidney
Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the International Society of Nephrology. This is an open
access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
chapter 1 www.kidney-international.org
S174
Kidney International (2024) 105 (Suppl 4S), S117–S314

serious study limitations. The certainty of the evidence for
mortality was further downgraded because there were few
events reported. The certainty of the evidence for perirenal
hematomas was downgraded because there was significant
statistical heterogeneity in the results across studies. The ERT
did not identify any studies that reported on the critical
outcome of retroperitoneal hemorrhage.
Values and preferences. The Work Group judged that many
people with CKD would choose to undergo a kidney biopsy
to establish the cause of their CKD more accurately and
potentially offer prognostic information. Thus, this recom-
mendation puts a high value on the specificity of a kidney
biopsy for the evaluation of cause as well as the very low
certainty evidence demonstrating a low risk of complications
associated with kidney biopsy. Because the potential that the
information gleaned from the biopsy may not directly or
immediately benefit the person, the Work Group jud ged that
some people may prefer to decline a kidney biopsy. The de-
cision to pursue biopsy should be a shared decision and be
informed by probability of and utility of the information
obtained on both diagnostic and prognostic fronts.
Resource use and costs. Resources available for evaluation
of cause w ill vary worldwide and is dependent on the
healthcare systems. People with CKD may not be able to pay
for biopsy or afford the time away from work for the pro-
cedure. Resources in specific countries may not permit
appropriate analysis of the obtained samples. Thus, healthcare
providers’ decisions to perform a kidney biopsy in the pres-
ence of limited resources may therefore be influenced based
on expected yield for that individual and the perceived value
of the extra information gained.
Considerations for implementation. To optimize benefit and
safety, a standardized approach for kidney biopsy with a
vetted and standardized operating protocol designed for local
implementation is warranted. Of note, most studies reported
using ultrasound-guided biopsies and older literature sug-
gesting higher bleeding rates were conducted in the absence
of guided biopsies; thus, we might infer that there is a po-
tential for higher rate of harms in “blind”/unguided biopsies.
Rationale
Kidney biopsy is an important par t of the investigations for
the cause of CKD. It is often deferred because of the potential
for harm or lack of recognition of potential utility. The evi-
dence to support safety of biopsy is heterogeneous and
therefore uncertain, but in the studies evaluated, appears to
confer low risk of harm, supporting our suggestion that
kidney biopsies should be considered when it is thought that
they can provide information to identify cause, facilitate
prognostication, and inform treatment strategies.
Special considerations
Pediatric considerations.
Children and young people with
kidney failure are more likely to have a genetic cause of their
disease than adults. In so me healthcare settings, genetic
testing may be pursued first, obviating the need for kidney
biopsy and the associated risks, which may be different in
children than adults.
1.2 Evaluation of GFR
The kidney has many functions, including excretory, endo-
crine, and metabolic functions. GFR is one component of
Centers of expertise
(multidisciplinary teams)
All nephrology
clinics
Connections with geneticists
and genetic counselors
Number of nephrologistsClinic type Knowledge level
High
Medium
Basic
As many
as possible
Few
All
Source of knowledge
Advanced training and
subspecialties and extensive
clinical experience
CME courses, workshops
and heuristically based
Medical school/
fellowships/licensing
Figure 10 | Proposed organization for implementing genetics in nephrology. Within a health system, multiple center types, provider
specialties, and education strategies are needed for optimal implementation of genetics in nephrology. A 3-tiered organization model includes
the following: (i) a basic, common level of knowledge in genetics among all nephrologists; (ii) clinical connections between nephrologists and
geneticists and genetic counselors; and (iii) centers of expertise where nephrologists with genetic expertise collaborate with geneticists and
genetic counselors. CME, continuing medical education. Reproduced from KDIGO Conference Participants. Genetics in chronic kidney disease:
conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2022;101:1126–1141.
100
Copyright ª 2022, Kidney Disease: Improving Global Outcomes (KDIGO). Published by Elsevier Inc. on behalf of the International Society of
Nephrology. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S175

excretory function but is widely accepted as the best overall
index of kidney function because it is generally reduced after
widespread structural damag e and most other kidney func-
tions decline in parallel with GFR in CKD.
In this section, we describe the overall approach for the
evaluation of GFR. As in the previous KDIGO Clinical
Practice Guideline for the Evaluation and Management of
Chronic Kidney Disease,
1
the first method to evaluate GFR
should be eGFRcr. If necessary for greater accuracy, the
approach then recommends subsequent supportive tests
from either the more accurate eGFRcr-cys or measurement
of GFR using urinary or plasma clearance of exogenous
filtration markers. In contrast to the previous guideline, we
emphasize the use of eGFRc r-cys based on accumulating
evidence for its greater accuracy across populations and the
use of mGFR given the known residual errors in all
estimating equations. We also describe laboratory
techniques and standards that satisfy the requirements for
robust result reporting. We encourage healthcare providers
to have a clear understanding of the value and limitations
of both filtration markers and mGFR, the importance of
standardization of assays for creatinine and cystatin C, and
quality control procedures for exogenous markers. Finally,
we describe cur rently available, validated estimating
equations that can be used for the reporting of GFR by
clinical laboratories.
1.2.1 Other functions of kidneys besides GFR
Practice Point 1.2.1.1: Use the term “GFR” when referring
to the specific kidney function of glom erular fi ltration. Use
the more general term “kidney function(s)” when dealing
with the totality of functions of the kidney.
The kidneys play several roles in the body, including
metabolism and excretion of substances, volume and BP
regulation, er ythropoietin production, and regulation of
electrolytes, acid-base status, and mineral homeostasis.
Glomerular filtration is one of many functions of the kidney.
GFR is considered the best overall assessment of kidney
functions as, in general, losses of these other functions
correlate with the loss of GFR. The term “kidney function”
reflects the entirety of different and complex physiological
functions of the kidney ; thus, kidney function should not be a
term used interchangeably with GFR.
Assessment of the overall functions of the kidney is a
complex task. GFR is used as the primary tool to assess kidney
function in practice. Loss of other kidney functions are
known as complications of CKD and are addressed in
Chapter 3. This section focuses on how GFR can be evaluated
using both mGFR and eGFR.
Special considerations
Pediatric considerations.
There are numerous kidney dis-
orders in children that may present with tubular dysfunction
(e.g., Bartter’s and Dent disease) rather than decreased GFR
or albuminuria. These pr imarily result in polyuria and/or
electrolyte disturbances and may or may not progress to
reduced GFR or kidney failure. Thus, the exclusive use of GFR
in diagnosing CKD would not be of value in children, high-
lighting the importance of appreciating different markers
linked to different kidney functions.
1.2.2 Guidance to physicians and other healthcare providers
We describe a framework for evaluation of GFR beginning
with an initial test and followed by additional supportive tests
(Figure 11, Tables 7 and 8
127–142
).
Figure 11 depicts an algorithm for evaluation of GFR from
initial test using eGFRcr, followed by decisions for when to
perform supportive tests such as cystatin C or mGFR
(Tables 7 and 8). Healthcare prov iders should consider both
potential sources of error in eGFR as well as whether the
clinical decision requires a highly accurate GFR when
considering the need for additional tests. The level of
accuracy that is needed for a clinical decision for the use of
potentially toxic medications, a medication with a narrow
therapeutic window, or for other therapies with potential
for adverse events, may exceed the capability of any eGFR
equation, and in such cases, mGFR should be performed.
This assessment would ideally be performed every time a
GFR value is used to make a clinical decision.
Practice Point 1.2.2.1: Use serum creatinine (SCr) and
an estimating equation for initial assessment of GFR
(Figure 11).
There are no RCTs to quantify the impact for the use of
less accurate methods versus more accurate methods of
assessment of GFR. For most clinical circumstances, esti-
mating GFR from SCr is appropriate for diag nosis, stagin g,
and monitoring the progression of CKD, and observational
data documented an increase in CKD recognition and referral
to nephrologists shortly after the implementation of reporting
of eGFR by clinical laborator ies, especially for females and
elderly people.
143–145
GFR is used in many routine and
complex clinical decisions as an assessment of excretory
kidney function (Table 4) to detect and stage AKD and CKD,
determine CKD progression, dose medications, determine
appropriate use of diagnostic tests, and guide treatment
decisions around KRT therapies. Equations are available
that estim ate GFR using SCr and adjusting for sex and age,
and professional societies throughout the world have
recommended that GFR estimates should be used in
association with SCr reporting . Sources of error in GFR
estimation from SCr concentration include non–steady-state
conditions, non-GFR determinants of SCr, measurement
error at higher GFR, and interferences with the creatinine
assays. GFR estimates are less precise at higher GFR levels
than at lower levels, and healthcare providers should remain
aware of caveats for any estimating equation that may
influence the accuracy in an individual person.
chapter 1 www.kidney-international.org
S176
Kidney International (2024) 105 (Suppl 4S), S117–S314

Most people with CKD and their healthcare pr oviders would
prefer the more accurate assessment of kidney function resulting
from the use of GFR estimating equations compared with SCr
alone. Minimal cost or resources issues are expected because
creatinine is available in healthcare settings globally, and evalu-
ating GFR with the use of creatinine in the form of GFR esti-
mating equations has been recommended for >20 years.
eGFR from creatinine is widely used. Attention is required
to implement and ensure the quality of eGFR reporting by
clinical laboratories and ensure coordination with the elec-
tronic medical record (EMR), including those eGFR reports
from point-of-care settings (Section 1.2.2).
Recommendation 1.2.2.1: We recommend using
eGFRcr-cys in clinical situations whe n eGFRcr is less
accurate and GFR affects clinical decision-making
(Table 8
127-142
) (1C).
This recommen dation places a high value on using estimates of
GFR derived from a combination of creatinine and cystatin C in
clinical situations where eGFRcr is an unreliable or inadequate
assessment of GFR. There is consistent evidence that eGFRcr-cys
provides more accurate estimates of mGFR than eGFRcr and
eGFRcys in ambulator y people.
Initial test – eGFRcr*
Consider sources of error and need for
more accurate assessment.
Is eGFR thought to be accurate?
Consider potential sources of error in eGFRcr-cys
and need for an even more accurate assessment.
†
Is a more accurate assessment needed?
NoYes
No Yes
Measure
cystatin C
Evaluation of GFR for clinical application
15 30 45 60 90 120
Use eGFRcr
Measure
GFR
Use
GFRcr-cys
‡
Figure 11 | Approach to glomerular filtration rate (GFR) evaluation using initial and supportive tests. The algorithm describes the
approach to the evaluation of GFR. The approach uses initial and supportive testing to develop a final assessment of true GFR and to apply it in
individual decision-making. The initial test for the evaluation of GFR is creatinine-based estimated GFR (eGFRcr), which will be available for most
people because creatinine is measured routinely as part of the basic metabolic panel. If eGFRcr is expected to be inaccurate, or if a more
accurate assessment of GFR is needed for clinical decision-making, such as diagnosis or staging of chronic kidney disease or drug dosing, then,
if available, cystatin C should be measured, and creatinine and cystatin C–based estimated GFR (eGFRcr-cys) should be estimated. If eGFRcr-cys
is expected to be inaccurate, or if an even more accurate assessment of GFR is needed for clinical decision-making, then, if available, GFR should
be measured using plasma or urinary clearance of exogenous filtration markers. *Initial test may be estimated GFR by cystatin C (eGFRcys or
eGFRcr-cys) in otherwise healthy populations with changes in creatinine generation due to non-GFR determinants such as changes in muscle
mass or creatinine secretion or extrarenal elimination due to the use of specific medications.
†
Sources of error in eGFRcr-cys include very low
muscle mass or very high levels of inflammation, high catabolic states, or exogenous steroid use.
‡
Consider eGFRcys rather than eGFRcr-cys in
otherwise healthy populations with decreased creatinine generation due to reduced muscle mass or decreased creatinine secretion or
extrarenal elimination due to the use of specific medications.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S177

Key information
Balance of benefits and harms.
Please see Practice Point
1.2.2.1 regarding the benefit of accurate assessment of GFR for
clinical decision-making. In clinical practice, there may be
situations where the estimation of GFR from SCr alone may
be a source of error, for example, muscle wasting/loss, or
where greater accuracy of GFR estimation is required for
clinical decision-making (e.g., drug dosing). In most of these
situations, estimating GFR using a combined creatinine and
cystatin C equation provides the required degree of accuracy
and obviates the need for expensive and time-consuming
measurement of GFR using an approved gold standard
methodology. GFR estimating equations that incorporate both
creatinine and cystatin C have particular benefit in terms of
improved accuracy in relation to mGFR compared with
equivalent equations using only one of these markers.
91,92,146–149
In 2 large-scale studies in pooled cohorts of general pop-
ulation cohorts and clinical populations in North America
and Europe, the P
30
(defined as the percentage of the eGFR
values within 30% of mGFR) using eGFRc r-cys is in the
range of 90%,
91,147
which is considered optimal.
1
Greater
accuracy of eGFRcr-cys compared with eGFRcr or eGFRcys
is also observed in studies evaluating GFR estimating
equations compared with mGFR in other countries such as
Brazil, Congo, Pakistan, Singapore, Japan, and China, with
P
30
estimated between 80% and 90%,
77,78,83,88,93,136,150–154
which is considered adequate for most decision-making.
1
Potential harms include increased costs, as described
below, and greater complexity in the in terpretation of GFR
with discrepant results between eGFRcr, eGFRcys, and
eGFRcr-cys. This in turn may lead to an increased number of
nephrology consults, especially initially as healthcare pro-
viders may be unfamiliar with these new tests.
Certainty of evidence. The Work Group considered the
overall certainty of the evidence to be moderate to high in
ambulatory people who were neither frail nor had acute or
chronic illn esses, and low in other populations due to in-
consistencies and imprecision in the studies currently avail-
able in the literature. Most of the studies used in the
development and in itial external validation of these equations
were performed in ambulatory people who were neither frail
nor had acute or chronic illnesses. There remains a paucity of
studies examining the accuracy of eGFR in such pop-
ulations.
142
Many studies that have been performed in such
populations are small, increasing risk for analytical
variability, and show inconsistent results among the studies
even within the same disease. Some reports in populations
with cancer, HIV, or obesity demonstrate greater accuracy
for eGFRcr-cys than either eGFRcr or eGFRcys.
132–135,155–157
Consistent with these findings, a large study of people liv ing
in Stockholm, Sweden referred for an mGFR test who had
diagnoses for heart failure, liver failure, cancer, CVD, or
diabetes found eGFRcr-cys to be the most accurate and
least biased.
82
In other studies of sick or frail people, such
as very advanced liver or heart failure or those admitted to
the intensive care unit, all eGFR tests demonstrated very
low levels of accuracy.
73,137,158–161
There are insufficient data to indicate the accuracy of
eGFRcr, eGFRcys, or eGFRcr-cys for many diseases. For
example, in people with high cell turnover such as hematologic
cancers, we expect that cystatin C would provide highly inac-
curate estimates due to the increase in cystatin C because of cell
turnover rather than decreased GFR disease.
162–165
However ,
there are no data to evaluate that hypothesis. Importantly, even
for people from populations where eGFRcr-cys has been
demonstrated to be more accurate, healthcare providers should
assess the potential sources of error in eGFR and the need for a
highly accurate level of GFR. Among people who are frail or
with multiple comorbid illnesses, eGFRcr-cys may be insuffi-
ciently accurate due to large contributions from non-GFR
determinants of creatinine, cystatin C, or both markers.
Conversely, in otherwise healthy populations with decreased
Table 7 | Description of initial and supportive tests for the evaluation of GFR
GFR assessment method Specific tests Guidance for use and implementation
Estimated GFR Creatinine (eGFRcr) Most used method to assess GFR. In most cases,
initial test for the evaluation of GFR.
Standardized assay required to decrease between-center
analytical variation
Cystatin C (eGFRcr-cys, eGFRcys) Used in selected circumstances as listed in Table 8
Standardized assay required to decrease between-center
analytical variation
mGFR Gold standard. Urinary or plasma clearance of exogenous
markers (e.g., iohexol, iothalamate,
51
Cr-EDTA, and
99m
Tc-DTPA)
Used in selected circumstances as listed in Table 8
Standard protocols for clearance methods and for the
standardized assay
Timed urine clearance Creatinine Highly prone to errors and recommended only when
no other options for supportive tests for GFR evaluation;
performance under supervised conditions may decrease error
Nuclear medicine
imaging
Imaging of the kidneys after injection of tracer cleared
by the kidneys (e.g.,
99m
Tc-DTPA scintigraphy)
Highly prone to errors; not recommended
51
Cr-EDTA, chromium 51-labeled ethylenediaminetetraacetic acid;
99m
Tc-DTPA, technetium 99m-labeled diethylenetriamine pentaacetate; eGFRcr, creatinine-based
estimated GFR; eGFRcr-cys, creatinine and cystatin C–based estimated GFR; eG FRcys, cystatin C–estimated GFR; GFR, glomerular filtration rate; mGFR, measured glomerular
filtration rate.
chapter 1 www.kidney-international.org
S178
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table 8 | Indications for use of cystatin C
Domain Specific clinical condition Cause of decreased accuracy Comments on GFR evaluation
Body habitus and changes
in muscle mass
Eating disorders
127
Non-GFR determinants of SCr eGFRcys may be appropriate if no comorbid illness
other than reduction in muscle mass.
Extreme sport/exercise/
body builder
Non-GFR determinants of SCr eGFRcys may be appropriate if an increase in muscle
mass is the only abnormality.
Above-knee amputation
128
Non-GFR determinants of SCr eGFRcys may be appropriate in those without other
comorbid conditions. Suggest eGFRcr-cys in
those with comorbid illness.
Spinal cord injury with
paraplegia/paraparesis or
quadriplegia/quadriparesis
Non-GFR determinants of SCr eGFRcys may be appropriate in those without other
comorbid illness. Suggest eGFRcr-cys in those
with comorbid illness.
Class III obesity
a,b
Non-GFR determinants of SCr
and SCys
eGFRcr-cys demonstrated to be most accurate.
Lifestyle Smoking
129-131
Non-GFR determinants of SCys Minimal data, suggest eGFRcr if no changes to non-
GFR determinants of SCr or comorbid illness.
Diet Low-protein diet Non-GFR determinants of SCr
Minimal data, suggest eGFRcr may be appropriate if
no changes to non-GFR determinants of SCr or no
comorbid illness.
Keto diets Non-GFR determinants of SCr
Vegetarian Non-GFR determinants of SCr
High-protein diets
and creatine supplements
Non-GFR determinants of SCr
Illness other than CKD Malnutrition Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
eGFRcr-cys may be less accurate because of
coexistence of malnutrition and inflammation.
Suggest using mGFR for treatment decisions
based on the level of GFR.
Cancer
a,132-137
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
eGFRcr-cys demonstrated to be most accurate in
populations studied but likelihood of lesser
accuracy in more frail people or in cancers with
high cell turnover. Suggest using mGFR for
treatment decisions based on the level of GFR.
Heart failure
a,138,139
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Although limited data, eGFRcys appears less biased
but all have low accuracy. Suggest using eGFRcr-
cys or eGFRcys for routine GFR evaluation. Suggest
using mGFR for treatment decisions based on the
level of GFR.
Cirrhosis
a,79,140,141
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Although limited data, eGFRcys appears less biased
but all have low accuracy. Suggest using eGFRcr-
cys or eGFRcys for routine GFR evaluation. Suggest
using mGFR for treatment decisions based on the
level of GFR.
Catabolic consuming
diseases
c
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Minimal data but eGFRcr-cys may be inaccurate.
Suggest using eGFRcr-cys vs. eGFRcr for routine
GFR evaluation. Suggest using mGFR for treatment
decisions based on the level of GFR.
Muscle wasting diseases
142
Chronic illness, presumed
impact on non-GFR
determinants of SCr and SCys
Minimal data. One study shows large bias for both
eGFRcr and eGFRcys. Suggest using eGFRcr-cys
for routine GFR evaluation. Suggest using mGFR
for treatment decisions based on the level of GFR.
Medication effects Steroids (anabolic, hormone) Non-GFR determinants of SCr.
Effect on SCys not known
Physiological effect on SCys unknown, suggest
eGFRcr-cys.
Decreases in tubular secretion Non-GFR determinants of SCr eGFRcys may be appropriate if medication affects only
creatinine and no comorbid illness. Suggest using
mGFR for treatment decisions based on the level
of GFR.
Broad spectrum antibiotics
that decrease extrarenal
elimination
Non-GFR determinants of SCr eGFRcys may be appropriate if medication affects only
creatinine and no comorbid illness. Suggest using
mGFR for treatment decisions based on the level
of GFR.
eGFR, estimated glomerular filtration rate; eGFRcr, creatinine-based estimated GFR; eGFRcr-cys, creatinine and cystatin C–based estimated GFR; GFR, glomerular filtration rate;
mGFR, measured glomerular filtration rate; SCr, serum creatinine; SCys, serum cystatin C.
a
Data summarized in Adingwupu et al.
149
b
Obesity class III varies by region but commonly body mass index >40 or >35 kg/m
2
.
c
Catabolic consuming disease may include tuberculosis, AIDS, hematologic malignancies, and severe skin diseases. There are no data with measured glomerular filtration rate
(mGFR) to evaluate this directly.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S179

creatinine generation due to reduced muscle mass or decreased
creatinine secretion or extrarenal elimination due to the use of
specific medications, it is possible that eGFRcys rather than
eGFRcr-cys would be preferred.
Values and preferences. The Work Group judged that most
people and most healthcare providers would want to use the
most accurate assessment of GFR available to them and
would, therefore, wish to estimate GFR from a combination
of creatinine and cystatin C, when available. However, they
would also balance additional costs associated with cystatin C
against the potential benefits.
Differences between eGFRcr and eGFRcys may prompt
recognition that both are estimates of GFR and both are asso-
ciated with error, requiring interpretation as to the best esti-
mate of GFR. In our view, this is desirable, and uncertainty as to
the level of GFR is an indication for nephrology referral.
Resource use and costs. Costs for the higher frequency of
cystatin C testing include one-time costs associated with initi-
ation of the assay within a laboratory, which include building
the information technology infrastructure and method verifi-
cation studies, and continuous costs associated with main-
taining the assay, which include reagents, daily quality control,
requirements for calibration verification, and proficiency
testing. Reagent costs are currently more expensive than
creatinine but are lower compared with other commonly used
biomarkers. If cystatin C is performed in an outside laboratory,
other costs, as with any laboratory test, may ensue. Additional
costs may also be a result of the increased referrals to ne-
phrologists to assist the inter pretation of potentially discordant
results between eGFRcr and eGFRcys. Ideally, these decrease
over time with increased utilization.
Considerations for implementation. We recognize that for
these recommendations to be implemented, cystatin C needs
to be widely available. Wherever possible, access to both
creatinine and cystatin C measurements should be made
available when evaluating GFR. Education for healthcare
providers and people with CKD for optimal use and inter-
pretation of these tests is required. See Sect ion 1.2.3 for details
regarding the measurement of creatinine and cystatin C by
clinical laboratories.
Rationale
We describe a framework for the evaluation of GFR beginning
with an initial test and followed by additional supportive tests
(Figure 11, Table 7). Cystatin C is an alternative endogenous
filtration marker that is now increasingly available. Its assay
can be put on autoanalyzers, and therefore its utilization could
be increased with clinical demand. eGFRcr-cys provides the
most accurate estimate and is recommended as the primary
supportive test for people in whom there are concerns about
eGFRcr accuracy (Table 8
127–142
). However, there remain
residual errors with some groups of people having a very high
level of errors. In such people, we advocate using mGFR. We
anticipate that such considerations be made at every encounter
where GFR is being used for a clinical decision.
Practice Point 1.2.2.2: Where more accurate ascertainment
of GFR will impact treatment decisions, measure GFR using
plasma or urinary clearance of an exogenous filtration
marker (Table 9).
Given the benefit of accurate assessment of GFR for clin-
ical decision-making, there is a need to appreciate the value
and circumstances in which directly mGFR is required. The
greatest benefit of mGFR is that it is less influenced by non-
GFR determinants, in contrast to eGFR. GFR is measured
using exogenous filtration markers and urinary or plasma
clearance. The precision of mGFR can be determined from
variability with repeated measures. Time-to-time variability is
the method used to assess error.
One systematic review summarizing the available data
comparing current GFR measurement methods to each other
and to the classic gold standard of inulin urinary clearance rec-
ommended the use of iothalamate, iohexol, ethyl-
enediaminetetraacetic acid (EDTA), and diethylenetriamine
pentaacetate (DTPA) as exogenous markers of choice.
166
A
subsequent study recommended against plasma
99m
Tc-DTPA,
especially when clearances are performed over 2–4hours.
167
Several studies demonstrate that the method by which the
clearance of exogenous markers is measured may impact
accuracy. For example, for people with lower GFRs, delayed
blood sampling is most accurate, whereas for people with
Table 9 | Comparison of estimated GFR and measured GFR
Estimated GFR by SCr and/or cystatin C Measured GFR
Inexpensive and easy to implement More expensive, more time-consuming, and invasive
Widely available and may also be used
at point of care, easily repeatable
Only available in certain centers
Methods to measure that do not require urine collections are available (i.e., plasma clearance)
Most protocols require repeat blood samples potentially over a long duration
Microsampling tests by fingerpick enable point-of-care testing. Testing has been described,
but not routinely performed
Not sufficiently accurate and precise
for all clinical situations
Accurate for GFR in all situations and across the GFR range. Requires individualized protocols
Lags behind changes in GFR Able to identify early changes in GFR
Subject to non-GFR determinant confounding Less influenced by non-GFR determinants
GFR, glomerular filtration rate; SCr, serum creatinine.
chapter 1 www.kidney-international.org
S180
Kidney International (2024) 105 (Suppl 4S), S117–S314

better-preserved GFRs, earlier blood sampling is most accurate,
and in people with extensive edema or ascites, plasma clearance
protocols are very inaccurate and not recommended; instead,
urinary clearance protocols are recommended.
167
Finally, it is
well recognized that assessing GFR using the imaging of
nuclear tracers is less accurate than eGFR, and we do not
recommend it as a method to measure GFR.
168
The evaluation of time-t o-time variability of plasma
clearance of iohexol and eGFR found a within-subject bio-
logical coefficient of variation (CV) for mGFR of 6.7% (95%
CI: 5.6%–8.2%), whereas CVs for eGFRcr, eGFRcys, and
eGFRcr-cys were approximately 5.0%.
169
Other studies have
observed CV for this same mGFR method ranging from
approximately 5% to 10%.
169,170
There are less data for
other methods; for urinary clearance of iothalamate,
estimated CVs were 6.3% and 16.6% across 2 studies.
171–173
The Work Group judged that there will be some clinical
situations where estimating GFR from both creatinine and
cystatin C will be insufficiently reliable or precise, and the
greatest benefit and least harm will be achieved by measuring
GFR with the appropriate standardized methods.
Costs for mGFR are variable and harder to quantify. The
infrastructure required is greater, as testing requires both
patient and personnel time for inserting a peripheral intra-
venous catheter, administering the exogenous marker, col-
lecting serial blood specimens over several hours (depending
on the protocol), and the associated materials for the
collection and measur ing blood levels by high-performance
liquid chromatography or mass spectrometry. Utilization of
mGFR may require input from a nephrologist in some set-
tings, which would also add to the costs of testing.
All nephrologists ideally should therefore have access to at
least 1 method to measure GFRusing plasma or urinary clearance
of exogenous markers. To ensure highly accurate measurements,
these clearance methods should be performed using standard
operating procedures. External quality assessment (EQA) should
be used for assays of the exogenous markers. Special consider-
ations in clearance methods are required for some populations to
obtain a high level of accuracy (e.g., later sampling time for
people with low GFR or urinary, instead of plasma clearance for
edematous people). GFR centers, under the direction of a
nephrologist champion or laboratory director, analogous to
cardiac imaging, are likely to help both increase utilization and
ensure high quality results. There will be additional requirements
for storage, administration, and disposal if radionuclide meth-
odologies are adopted. National kidney societies can work with
payers to support reimbursement for mGFR procedures. The
European Kidney Function Consortium (EKFC) together with
the European Federation of Clinical Chemistry and Laboratory
Medicine is currently harmonizing mGFR protocols for iohexol
plasma clearance to deliver standardized operating procedur es
for GFR measurements in the near future.
Decisions to measure GFR should be made by both ne-
phrologists and other physicians using the framework sug-
gested in Figure 11. Physicians should determine how accurate
the GFR needs to be for a specific clinical decision. If greater
accuracy is needed than can be achieved using eGFR, mGFR
is recommended. Greater accuracy may be required due to
inaccuracy of eGFR in the individual perso n due to the
presence of non-GFR determinants or due to the requirement
of the clinical setting. Table 10 lists indications for when one
might consider mGFR as opposed to eGFRcr-cys.
We describe a framework for the evaluation of GFR beginning
with an initial test and followed by additional supportive tests
(Figure 11, Table 7). mGFR is recommended when there are
concerns about the accuracy of eGFRcr-cys (Table 8
127-142
)or
where an accurate level of GFR is required for optimal
decision-making, as described in Tabl e 10.
Practice Point 1.2.2.3: Understand the value and limitations
in both eGFR and measured glomerular filtration rate
(mGFR) as well as the variability and factors that influence
SCr and cystatin C measurements.
All studies evaluating the performance of eGFR compared
with mGFR observe error in any GFR estimate. Even in
populations where there is a high accuracy (i.e., P
30
of 90%),
10% of the population would have errors $30% relative to
mGFR. Within these studies, error rates are likely to be higher
in some subgroups and lower in others. A critical component
of the recommended approach to evaluation of GFR (Figure
11) is that physicians have a clear understanding of the
value and limitations of eGFR and mGFR, which defines
when a person requires one or another supportive test.
The source of error in eGFR may be related to errors in eGFR
or in mGFR (Figure 12
174
). The most important sources of error
are non-GFRdeterminants of either creatinine or cystatin C. The
non-GFR determinants of creatinine include generation by diet
and muscle mass, tubular secretion, and extrarenal
elimination.
130,175
The non-GFR determinants of cystatin C
are less well understood but thought to be higher adiposity,
smoking, hypo- and hyperthyroidism, glucocorticoid excess,
and chronic inflammation (as indicated by insulin resistance,
higher levels of C-reactive protein and tumor necrosis factor,
or lower levels of serum albumin).
129,130,176–185
Table 10 | Indications for measured GFR
Clinical conditions in which eGFRcr-cys is inaccurate or uncertain due to potential non-GFR determinants of creatinine and cystatin C. This may include
catabolic states, such as serious infections or inflammatory states, high cell turnover as in some cancers, advanced cirrhosis or heart failure, use of high-
dose steroids, or the very frail. See Figure 12 for approach to individual decision-making.
Clinical settings in which greater accuracy is needed than is achieved with eGFRcr-cys. For example, decisions about simultaneous kidney transplantat
the time of other solid organ transplant, kidney donor candidacy, and drug dosing if there is a narrow therapeutic index or serious toxicity (e.g.,
chemotherapies that are cleared by the kidney).
eGFRcr-cys, estimated GFR by creatinine and cystatin C; GFR, glomerular filtration rate.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S181

mGFR also differs from the true physiological GFR, which
itself cannot be directly measured. Errors may be related to
analytical errors in the assay or the clearance procedure. For
example, the overestimation of GFR is seen if late samples are
not taken for people with low GFR.
167,186
Urinar y clearances
are preferred to plasma clearance methods in people with
extensive third spacing of fluid.
169–173
In the absence of
changes related to disease progression, a change in mGFR
from time to time may occur due to preanalytical (e.g.,
patient preparation and time of day), analytical (laboratory
measurement variability), and biological (changes in true
physiological GFR) variabilit y, as is the case for eGFR. This
does not detract from the advantage of mGFR as being free
from non-GFR determinants. It is important for
nephrologists to appreciate and understand these errors and
nuances to appropriately order the right tests in specific
circumstances.
Practice Point 1.2.2.4: Interpretation of SCr level requires
consideration of dietary intake.
Most studies measuring GFR for clinical or research pur-
poses are performed in the morning after a period of fasting or
moderate protein intake. Ideally, optimal application of eGFR
would simulate these conditions. Several studies have
documented the impact of a cooked meat or fish meal on
creatinine concentrations.
187
For example, one study
demonstrates increase in SCr of approximately 20
m
mol/l
(0.23 mg/dl) which in the study population was equivalent to
decrease in eGFR of approximately 20 ml/min per 1.73 m
2
.
Maximum postprandial effects were reached in some subjects
by 2 hours and others by 4 hours. Waiting for at least 12
hours before the measurement of SCr, after meat or fish
intake, best avoids this effect. We recognize that this approach
may be challenging to implement in the clinical environment.
Practice Point 1.2.2.5: Assess the potential for error in
eGFR when assessing a change in GFR over time.
When evaluating a change in eGFR over time, the question
is whether the true GFR is changing. However as described
above, there are several other potentia l causes for a change in
observed eGFR, other than AKI, such as changes in non-GFR
determinants of the filtration markers or analytical errors in
the assays. Healthcare providers should consider whether
there has been a change in non-GFR determinants (e.g., a
recent meat meal now or at the first measurement or change
in muscle mass or extreme activity). The impact of the
combined effect of analytical and biological variation on
eGFR in determining progression is discussed in Chapter 2.
Biological variability Analytical variability
Non ideal properties of
mGFR Measurement error in the
clearance procedure
Measurement error in the assay of
determinants of serum
concentrations of endogenous
eGFR Measurement error in the assay
mGFR P
15
~ 90%
± 15%
26–35
± 15%
51–69
15 30 45 60 90 120
eGFR P
30
~ 90%
± 30%
21–39
± 30%
42–78
Figure 12 | Sources and magnitude of error around measured glomerular filtration rate (mGFR) and estimated GFR (eGFR). It is
important to determine how accurate the assessment of GFR needs to be for clinical decision-making. P
30
for eGFR refers to the percent of
eGFRs that are within 30% of mGFR. If accuracy within 30% is acceptable (P
30
>80%) or optimal (P
30
>90%), eGFR may be sufficient, provided
that there are not large deviations in non-GFR determinants of creatinine or cystatin C. If greater accuracy is needed, mGFR is advised. The
accuracy for mGFR is based on time-to-time variability. P
15
for mGFR refers to the percent of one mGFR that was within 15% of the second. At a
GFR of 60 ml/min per 1.73 m
2
, 30% accuracy for eGFR corresponds to 42 to 78 ml/min per 1.73 m
2
and 15% accuracy for mGFR corresponds to
51 to 69 ml/min per 1.73 m
2
. At a GFR of 30 ml/min per 1.73 m
2
, 30% accuracy for eGFR corresponds to 21 to 39 ml/min per 1.73 m
2
and 15%
accuracy for mGFR corresponds to 26 to 35 ml/min per 1.73 m
2
. Non-GFR determinants of endogenous filtration markers include generation,
tubular handling, and extrarenal elimination. Nonideal properties of exogenous filtration markers include tabular handling and extrarenal
elimination. Reproduced from Kidney International, volume 96, issue 2, Inker LA, Levey AS. Knowing your GFR—when is the number not (exactly)
the number? Pages 280–282.
174
Copyright ª 2019, with permission from the International Society of Nephrology. Published by Elsevier Inc. All
rights reserved.
chapter 1 www.kidney-international.org
S182
Kidney International (2024) 105 (Suppl 4S), S117–S314

When evaluating a change in GFR using mGFR, the com-
bined effect of changes in biological and analytical variation
should be considered as part of the interpretation of the re-
sults (Figure 12
174
).
169
Practice Point 1.2.2.6: Consider the use of cystatin C–based
estimated glomerular filtration rate (eGFRcys) in some
specific circumstances.
The combination of eGFRcr and eGFRcys together is more
accurate than eGFRcr or eGFRcys alone.
91,147
The greater
accuracy is due to the fact that the non-GFR determinants
for each marker are different, and therefore using both
leads to convergence on the estimate of GFR and minimizes
the effect of either marker.
188
In individuals where non-GFR determinants of creatinine
or cystatin C are substantially greater than for the other
marker, eGFRcr-cys would not provide the more accurate
estimate. This imbalance is more likely to occur for creati-
nine, given its association with diet and muscle mass, which
can vary greatly across various people. In such cases, it would
be reasonable to use eGFRcys.
The non-GFR determinants for cystatin C are less well
studied, and it is erroneous to assume that eGFRcys provides
the more accurate estimate in all circumstances. We, there-
fore, advise limiting this strategy to selected clinical settings
where people are otherwise healthy with known changes in
non-GFR determinants of creatinine. For example, in 1 study
that compared eGFRcr and eGFRcys before and after ampu-
tation in otherwise healthy military veterans, there was a
sizable change in eGFRcr as would be expected with the loss
of a limb and loss of mobility, but no change in eGFRcys.
128
In another study of people with anorexia, serum levels of
cystatin C were more strongly correlated with mGFR than
were levels of SCr, but this has not been further evaluated
using eGFR and standardized assays.
127
Other situations
may be where there are medications that inhibit tubular
secretion of creatinine, although there are no studies to
provide evidence to drive guidance.
Practice Point 1.2.2.7: Understand the implications of dif-
ferences between eGFRcr and eGFRcys, as these may be
informative, in both direction and magnitude of those
differences.
For people who have simultaneous SCr and cystatin C
values, the agreement or discrepancy between eGFRcr and
eGFRcys may help to guide further actions. Several studies
have demonstrated that 25%–30% of people have discordance
between eGFRcr and eGFRcys as large as or larger than 15 ml/
min per 1.73 m
2
or $20%.
82,138,189
One study demonstrated
that factors associated with higher values for eGFRcr
compared with eGFRcys included older age, female sex,
non–Black race, higher eGFR, higher BMI, weight loss, and
current smoking.
190
Two recent studies demonstrate that
when there is concordance between eGFRcr and eGFRcys,
there is high and similar accuracy for eGFRcr, eGFRcys, and
eGFRcr-cys with estimated P
30
of 87%–91%.
82,138,189
In
contrast, when there is discordance, eGFRcr-cys is more
accurate than either eGFRcr or eGFRcys. This suggests that
when eGFRcr and eGFRcys are discordant, it is reasonable
to continue to measure cystatin C serially in addition to
creatinine in those settings where GFR will affect clinical
decisions. It is also reasonable to consider performing/
conducting mGFR when using medications with narrow
therapeutic index or high toxicity or to inform critical
treatment decisions (Chapter 4).
Practice Point 1.2.2.8: Consider timed urine collections for
measured creatinine clearance if mGFR is not available and
eGFRcr-cys is thought to be inaccurate.
mGFR is not available everywhere. In these settings, it
might be reasonable to consider measured urinary creatinine
clearance (CrCl). It is widely available and therefore
commonly used but is highly prone to error due to under- or
overcollection. A systematic review of GFR methods observed
a mean bias of 25% across 23 studies, and as such, did not
find this method to reach sufficient accuracy.
166
The errors
occur in both directions and thus do not appear so lely due
to the presence of tubular secretion of creatinine, which
would be expected to overestimate mGFR. For example, in
the pilot study for the African American Study of Kidney
Disease (AASK), 25% of participants had a 24-hour
measured CrCl that was at least 18% lower than the mGFR,
and another 25% had measured CrCl at least 23% greater
than the GFR. Of note, measured CrCl had substantially
better correlation with mGFR when it was measured during
an mGFR procedure
191
; therefore, if measured CrCl is to be
performed, then it should ideally be supervised given the
high risk of inaccuracy with urine collection.
Special considerations
Sex and gender considerations.
It is unclear how best to
estimate GFR in people who are transgender, gender-diverse,
or nonbinary, where a person’s gender identity is different
from their sex assigned at birth. Gender-affirming testos-
terone therapy is associated with an increase in SCr concen-
tration,
192
with less certainty for the impact of estrogen.
Gender-affirming testosterone therapy is associated with an
increase in serum cystatin C and gender-affirming estradiol,
and antiandrogen therapy is associated with a decrease in
serum cystatin C.
193
The impact of gender-affirming
hormone therapy, if any, on true GFR is unknown. In
keeping with guidance from the American Association of
Clinical Chemistry and the National Kidney Foundation,
194
evaluation of eGFR should use a shared decision-making
approach w ith the person with CKD, taking into account
muscle mass, sex horm one milieu , sex assig ned at birth,
and gender identity. We also note that the new EKFC
cystatin equatio n does not include a var iable for sex.
Pediatric considerations. There are currently insufficient
externally validated data to assess if combining creatinine and
cystatin improves the performance of pediatric eGFR equa-
tions. Internal analysis of the Chronic Kidney Disease in
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S183

Children (CKiD) cohort revealed that averaging the eGFRcr
and eGFRcys reduced mean bias in people who are Black,
White, and other race. Likewise, averaging eGFRs derived
from the equations improved accuracy to 89%–91% (as
assessed by P
30
) across race groups. This has not been
externally validated.
195
1.2.3 Guidance to clinical laboratories
Practice Point 1.2.3.1: Implement the laboratory standards
of care outlined in Table 11 to ensure accuracy and
reliability when assessing GFR using creatinine and
cystatin C.
Practice Point 1.2.3.2: Given available resources, clinical
laboratories may consider the possibility of measurement
of both creatinine and cystatin either as an in-house test or
as a referred test.
Consistency, standardization, and comparability of labo-
ratory measures of creatinine and cystatin C; the reporting of
results and of GFR estimates; and the flagging of reported
results where indicated are of paramount importance. The
assays used should have the required specificity for the ana-
lyte, and the calibration of assays is essential for the inter-
pretation of kidney function measures. Results should be
traceable to reference mater ials and methods listed on the
Joint Committee for Traceability in Laboratory Medicine
(JCTLM) database.
Estimation of GFR improves identification of CKD.
Adoption of the laboratory standards described here will
ensure that healthcare providers receive eGFR reports in a
consistent style and with assurance regarding the accuracy
and reliability of the result. Flagging decreased values for
eGFR can alert healthcare providers to the possibility of
kidney disease and may indicate the need for additional
evaluation or adjustment of doses of medications that are
excreted by the kidney.
Globally, most creatinine measurements are undertaken
using a colorimetric method (Jaffe). This method also reacts
with a variety of substances that are not creatinine (so-called
“non-creatinine chromogens,” e.g., glucose and acetoacetate),
typically comprising some 20% of the measured substan ce
reported as creatinine in adults at physiological creatinine
concentrations. Enzymatic assays are available that are more
specific for creatinine and less susceptible to che mical and
chromogenic (e.g., icterus and hemolysis) interferences.
Although enzymatic methods are not totally immune to the
interferences affecting the Jaffe method and may be suscep-
tible to other interferences specific to the enzymatic approach,
in the majority of people, use of an enzymatic method will
reduce the possibility of interference (Table 12
127,196–215
). It is
likely that cystatin C measurements will be less susceptible to
chemical and spectral interferences affecting creatinine assays,
but inevitably, interferences will surface with more extensive
clinical experience, for example, those due to circulating
antibodies that are seen with other immunoassays.
216–218
After venipuncture, in unseparated samples, there is a
gradual increase in measured SCr over time when the Jaffe
assay is used. This effect is not seen when enzymatic assays are
Table 11 | Implementation standards to ensure accuracy and reliability of GFR assessments using creatinine and cystatin C
Report eGFR in addition to the serum concentrations of filtration markers using validated equations.
Report eGFR rounded to the nearest whole number and relative to a body surface area (BSA) of 1.73 m
2
in adults using the units ml/min per 1.73 m
2
.
Reported eGFR levels <60 ml/min per 1.73 m
2
should be flagged as being low.
When reporting levels of filtration markers, report:
(i) SCr concentration rounded to the nearest whole number when expressed as standard international units (
m
mol/l) and rounded to the nearest
100th of a whole number when expressed as conventional units (mg/dl);
(ii) serum cystatin C concentration rounded to the nearest 100th of a whole number when expressed as conventional units (mg/l).
Measure filtration markers using a specific, precise (coefficient of variation [CV] <2.3% for creatinine and <2.0% for cystatin C) assay with calibration
traceable to the international standard reference materials and desirable bias (<3.7% for creatinine and <3.2% for cystatin C) compared with reference
methodology (or appropriate international standard reference method group target in external quality assessment [EQA] for cystatin C).
Use an enzymatic method to assay creatinine, where possible.
Separate serum/plasma from red blood cells by centrifugation within 12 hours of venipuncture.
When cystatin C is measured, measure creatinine on the same sample to enable calculation of eGFRcr-cys.
eGFR, estimated glomerular filtration rate; eGFRcr-cys, estimated glomerular filtration rate based on creatinine and cystatin C; GFR, glomerular filtration rate; SCr, serum
creatinine.
Table 12 | Reported examples of substances that may cause
analytical interferences in creatinine assays
Jaffe methods Enzymatic methods
Acetaminophen
196
Aspirin
196
Ascorbic acid
197
Bacterial contamination
198
Bilirubin
199,200
Blood-substitute products
201
Cephalosporins
202,203
Fluorescein
204
Glucose
205
Hemoglobin F
206
Ketones/ketoacids
207
Lipids
208
Metamizole protein
206-209
Pyruvate, including that arising
from delayed sample processing
143
Streptomycin
210
Bilirubin
211
Lidocaine metabolites
212
Metamizole
196
N-acetylcysteine
213
Proline stabilizers, present in
intravenous immunoglobulin
preparations
214
Phenindione
215
The nature of interference (magnitude and direction of bias) from the listed com-
pounds is dependent on the precise reaction conditions in use, in relation to timing
of spectrophotometric readings and chemical composition of the reagent: different
versions of the Jaffe and enzymatic methods used by different manufacturers will
respond in variable ways to interferences. Further information may be found in
Myers et al.
205
chapter 1 www.kidney-international.org
S184
Kidney International (2024) 105 (Suppl 4S), S117–S314

used.
219
We therefore advise that serum should be removed
from the red blood cells within 12 hours of venipuncture
when the Jaffe assay is being used.
As described in Section 1.2, eGFR is an imperfect estimate
of mGFR. At best, 90% of eGFR will fall within 30% of
mGFR. As shown in Figure 12, one of the sources of error
is analytical variability in measurement of the filtration
markers. Optimization of laboratory measurements of
creatinine and cystatin C can help to reduce the uncertainty
inherent in GFR estimation. The components of
measurement error that laboratories must address are
accuracy (trueness of the result), imprecision (analytical
variability of the result, commonly expressed as a CV), and
specificity (reduction of interferences in the measurement).
The availability of international reference standards for both
creatinine
220
and cystatin C
221
and demonstration that the
laboratory results have minimal bias compared with these
help to ensure the accuracy of results. Imprecision targets
are commonly based on the known biological variability of
biomarkers (https://biologicalvariation.eu/). Analytical
variability that is less than half the within-person biolog ical
variability is generally considered desirable.
222
The target
CVs proposed here for creatinine and cystatin C should be
achievable by automated laboratory methods. Achieving the
target precision and bias goals proposed will ensure that
laboratory error contributes to a less than 10% increase in
root mean square error when estimating GFR.
205
Most people with CKD, healthcare providers, and policy
makers would want laboratories to implement calibrated as-
says for creatinine and cystatin C that comply with interna-
tional standards and use reagents for analysis that conform to
internationally approved reference materials. Compliance
with the recommended standards would ensure confidence in
the results and in clinical decisions and any changes in
management and treatment made as a consequence.
Globally, most GFR estimates are currently produced using
creatinine results generated by Jaffe assays, which are rela-
tively inexpensive. Use of more specific enzymatic creatinine
assays can improve the estimation of GFR. However, enzy-
matic creatinine assays are more expensive than Jaffe assays.
Use of cystatin C in combined creatinine-cystatin C GFR
equations can also further improve GFR estimation, but
cystatin C measurement adds significantly to the cost.
Although the per-patient cost increase of enzy matic creatinine
and cystatin C measurement is relatively small, the imple-
mentation of these more expensive approaches has significant
cost implications across entire healthcare syste ms.
Implementation considerations include the following:
Creatinine. Resource limitations that may restrict access to
enzymatic creatinine should not be seen as a barrier to
implementation of a GFR reporting program based on Jaffe
creatinine measurement.
Cystatin C. Ideally, cystatin C will be available for timely
same-day results, which requires either measurement within
the local laboratory or alternatively as a referred test in
centralized laboratories. A range of commercially available
routine clinical biochemistry analyzers from a variety of
manufacturers can support cystatin C assays and will allow
turnaround time for results as rapid as that for routine
electrolytes and creatinine when provided locally. Timeliness
will affect utilization (i.e., if results are available on the same
day), then the test is more likely to be useful for routine or
urgent decisions, and this may increase the pressure on lab-
oratories to provide this test locally.
Estimated GFR. Implementation and modification (e.g., a
change in equation) of GFR estimation require close
communication between the laboratory and a range of clinical
users, including primary and secondary care healthcare pro-
viders, pharmacists, dietitians, and people with CKD.
223
Laboratories should only use GFR estimating equations that
have been sufficiently validated in the population to which
they are being applied and that are appropriate for the
creatinine and cystatin C assays in use (Sect ion 1.2.4).
223
They should also ensure that their end-to-end reporting
processes, including calculations embedded within the
laboratory information system, are subject to regular EQA.
Laboratory reports for computed values should indicate the
filtration marker (i.e., eGFRcr, eGFRcys, and eGFRcr-cys).
Documentation should indicate which equation was used.
To aid clarity in reporting across and within healthcare
systems, and to provide guidance regarding the number of
meaningful digits in a result, a standardized approach in
relation to reporting units of GFR, creatinine, and cystatin C
should be implemented. Input age may be rounded to whole
numbers or as a fractional year because the influence on
eGFR is small. To adjust GFR for differences in body size,
mGFR is commonly adjusted for body surface area (BSA),
with a population average BSA value of 1.73 m
2
being used. In
practice, eGFR values derived using most equations are
already adjusted for BSA, because BSA was taken into account
when the equations were originally developed using regres-
sion modeling against BSA-adjusted mGFR.
eGFR is mos tly computed using the information recorded
in the sex variable in EMRs. Some EMRs include legal sex, sex
assigned at birth, and gender identity, whereas others include
only one variable. In some cases, this variable may be missing
or reported as nonbinary. In these cases, eGFR values cannot
be computed and will be displayed as a missing value. Lab-
oratories should add a comment directing healthcare pro-
viders and people with CKD to online calculators to facilitate
a shared decision-making approach to the person with CKD.
The comment may also include a suggestion to use cystatin C
as there is less difference between eGFRcys values for males
and females and where there is now an option for computing
eGFR without the use of sex.
Together, the set of statements allow for a consistent
approach to the measurement and reporting of serum filtra-
tion markers and eGFR in clinical practice.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S185

Special considerations
Pediatric considerations.
Practice Point 1.2.3.3: Laboratories measuring creatinine in
infants or small children must ensure their quality control
process include the lowest end of the expected range of
values for the group of interest.
Practice Point 1.2.3.4: Consider the consistent use of
enzymatic creatinine assays in children, given the higher
relative contribution of non-creatinine chromogens to
measured creatinine in children when using the Jaffe assay,
and the high prevalence of icteric and hemolyzed samples
in the neonatal period.
Practice Point 1.2.3.5: An eGFRcr level <90 ml/min per
1.73 m
2
can be flagged as “low” in children and adolescents
over the age of 2 years.
In the KDIGO 2012 Clinical Practice Guideline for the
Evaluation and Management of Chronic Kidney Disease,
1
a
cutoff of 60 ml/min per 1.73 m
2
was chosen to define “low”
GFR for children. In this update, we advise increasing the
cutoff to 90 ml/min per 1.73 m
2
for children and adolescents.
In children and adolescents, a reduced GFR is likely to
deteriorate further and, therefore, warrants closer monitoring
and early intervention. Children with lower-than-normal GFR
often experience deterioration in GFR during periods of rapid
growth in adolescence.
224
Those with subnormal GFR during
adolescence are more likely to eventually experience clinically
important low GFR later in life. Even mild decreases in
eGFR (i.e., CKD G2) are associated with poor kidney
outcomes. In a US study of over 7 million children captured
by electronic health record data, 8600 had CKD G2. At 10
years from cohort entry, the rate of reaching kidney failure
or a 50% decline in eGFR ranged from around 10%
(nonglomerular CKD) to around 40% (glomerular CKD).
225
Furthermore, eGFR between 60 and 90 ml/min per 1.73 m
2
is sometimes associated with impaired linear growth and
with hyperparathyroidism in children and adolescents.
226,227
A higher cutoff defining low GFR for children and ado-
lescents also reflects their longer life expectancy. Early inter-
vention may have profound protection of GFR. CKD G2 has
long been considered to reflect decreased GFR in children,
reflected by the inclusion of children and adolescents with
CKD G2 in pediatric CKD trials and cohort studies, including
Effect of Strict Blood Pressure Control and ACE Inhibition on
the Progression of CRF in Pediatric Patients (ESCAPE),
228
Hypertension Optimal Treatment in Children with Chronic
Kidney Disease (HOT-KIDS; United Kingdom),
229
CKiD
(North America),
230
KoreaN cohort study for outcomes in
people with pediatric CKD (KNOW-PedCKD; South
Korea),
231
and the Kids with CKD (KCAD; Australia and
New Zealand).
232
The definition of CKD remains
unchanged; the flagging of GFR <90 ml/min per 1.73 m
2
as
low for children and adolescents reflects the need for closer
assessment for evidence of kidney damage and monitoring.
1.2.4 Selection of GFR estimating equations
Recommendation 1.2.4.1: We recommend using a
validated GFR estimating equation to derive GFR
from serum filtration markers (eGFR) rather than
relying on the serum filtration markers alone (1D).
Practice Point 1.2.4.1: Use the same equation within
geographical regions (as defined locally [e.g., continent,
country, and region] and as large as possible). Within such
regions, equations may differ for adults and children.
The recommendation places a high value on the use of an
estimating equation for GFR that has been validated in the
population of interest and which has been shown to be most
accurate in comparison with mGFR and a low value on the
comparison of performance characteristics across different
equations. The key points are to use an equation validated in
and most suited to the population of interest.
Key information
Balance of benefits and harms.
This recommendation rec-
ognizes that there are now a number of validated GFR esti-
mating equations available. They have differing performance
characteristics, which may differ depending on the population
of interest. The intention of suggesting the use of the same
equation within a region is to reduce clinical confusion if
people with CKD go to different laboratories within a region
and to enable appropriate populati on compari sons. Use of
different equations (and thus different eGFR values for the
same person) may lead to confusion for both the individual
person and their healthcare providers.
The Work Group judged that there is potential for harm if
people get different eGFR values when receiving care in
different settings. As described in Section 1.2.2,thereare
several sources of variability in eGFR. Differences between
valid equations are often substantially less than these sources
of variability, but that might not be understood by most
healthcare providers or people, leading to excessive anxiety
and repeated testing for small changes in GFR as related to
the use of a different GFR estimating equation. Using the
same equation within the same geographical region can
eliminate the source of variation that is related to the specific
parameters of the GFR estimating equation.
There is benefit to clinical care, research, and public health
with the use of validated equations such that decisions,
research findings, and public policy are informed by accurate
estimates of CKD.
Certainty of evidence. This recommendation is based on
Work Group consensus regarding good clinical practice to use
a GFR estimating equation validated in the population of
interest. Table 13 lists criteria for validated equations.
The criteria were developed by accumulated evidence from
assessment of the performance of eGFR versus mGFR across
equations and populations. For example, use of equations
developed using assays that are not traceable to reference
chapter 1 www.kidney-international.org
S186
Kidney International (2024) 105 (Suppl 4S), S117–S314

materials cannot be applied to settings with differences in
assays,
233
or use of equations developed in one population
may not perform well in other populations with very
different characteristics.
153,234,235
Values and preferences. There are now several valid equa-
tions that can be reasonably used in local settings. The Work
Group recognizes that different values and preferences may
lead to different decisions in selection among validated GFR
estimating equations. Thus, instead of being prescriptive, we
list a set of criteria that defines a valid equation, a set of
equations considered valid at this time, and a list of metrics to
define better versus worse performance as evaluated in the
local area. It is of value that GFR thresholds for definiti on and
staging be standardized using valid equations optimized for a
specific region helps to ensure this occurs. Where possible,
inclusion of representation from key constituents in the
population in the development of the equation and ensuring
that it remains valid in those populations is also of value.
Using validated eGFR equations improves the accuracy of
assessment of true GFR but remains imperfect, and no single
equation performs consistently across all populations. The Work
Group judged that people with CKD and their healthcare pro-
viders would want GFR estimated using the equation providing
the greatest accuracy in the population of their geographical
region. The Work Group recognizes that across the world there is
significant variation in the sociodemographic and ethnic
makeup of populations and that even well-validated equations
developed in different populations may not perform as well as
others developed and validated in the population of interest.
Resource use and costs. There are a number of initial costs
including human resource costs associated with taking the time
to decide on which equation, then time and technical infor-
mation resources to be considered to change the computation,
and the laboratory and nephrology teams to test the new
equation and inform the clinical partners on the change. In
addition, education for primary care providers, people with
CKD, and other healthcare providers is also required, which
incurs both direct and indirect costs. There will be costs, both
human resource and meetings costs, associated with decision-
making around which equation to use. Additional costs will be
accrued if validation and impact studies are required.
Considerations for implementation. Each region should have
a mechanism for review and selection of equations for
implementation by laboratories. For most countries, this
might be through the national kidney society working in
collaboration with laboratory physician organizations or
regional laboratory groups, as has occurred in the United
States and Europe, respectively.
236,237
Decisions at this level by
continental or national organizations are likely to minimize
the likelihood that decisions for equation use will be made
within small geog raphical areas or governed by local
decisions, leading to greater variation in eGFR and
uncertainty by people with CKD and healthcare providers.
Considerations in decisions about implementation will
reflect the balance of the criteria listed in Table 13.
There are likely to be tradeoffs between optimal accuracy
in local regions versus uniformity. Equations optimized for a
specific region can help to ensure that the GFR thresholds for
disease definition, classification, and risk estimation have the
same implications across regions. However, it would lead to
barriers to implementation, as it will not be possible for all
regions to conduct a sufficiently large and representative
study to evaluate these equations and develop modifications.
If not possible, or in the interim, we advise using equations
that were developed in populations most similar to the
available populations. For example, until more accurate re-
gion-specifi c equations are available in countries within
Central or South America, it may be reasonable to use CKD-
EPI given the inclusion of Black and Hispanic participants in
the development of equation, and within African countries, to
use the EKFC equations using the Q-values, the median SCr
concentration in a cohort developed in 2 African countries.
234
Table 13 | Criteria for a validated GFR estimating equation
Criteria Consideration
Developed using rigorous measured GFR (mGFR) methods; ideally using comparable measurements for
all individuals in the development populations
Development methods
Developed using assays for filtration markers traceable to reference materials with acceptable accuracy
and imprecision
Development methods
Developed with sufficient sample size for the population Development population
Study populations with a wide range of clinical characteristics and GFR, where possible representative
of the clinical populations in which equations are to be applied, including representative samples
of general population and people with kidney disease
Development population
Performance vs. mGFR evaluated in separate populations from that in which it was developed
(i.e., external validation, not random split of development data)
Accuracy
Performance shows certain thresholds for performance compared with other equations (see Table 15) Accuracy
Can be reported by laboratories (i.e., no other variables required for computation that are
not readily available)
Implementation by clinical laboratories
GFR, glomerular filtration rate.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S187

However, other considerations may also be relevant for the
regional organization making these decisions. We also note
that if cystatin C is available, then using eGFRcr-cys would
simplify the selection of the equation as the performance of
eGFRcr-cys computed from the different equations is more
similar than that of eGFRcr.
Rationale
The KDIGO 2012 Clinical Practice Guideline for the Evalu-
ation and Management of Chronic Kidney Disease recom-
mended “to report eGFRcr in adults using the 2009 CKD-EPI
creatinine equation. An alternative creatinine-based GFR
estimating equation is acceptable if it has been shown to
improve accuracy of GFR estimates compared to the 2009
CKD-EPI creatinine eq uation.” We are updating this recom-
mendation to accommodate the availability of alternative
equations that also have high levels of accuracy. Since the
publication of the KDIGO 2012 Clinical Practice Guideline
for the Evaluation and Management of Chronic Kidney Dis-
ease
1
for GFR estimation in adults, there have been 3 main
sources of validated equations: those developed by the
CKD-EPI, those developed by EKFC, and modifications of
each for use in specific regions (Table 14
83,91,147,148,235,238–
243
). Table 15 lists thresholds for key performance metrics
that can be used to guide comparison between equations.
The CKD-EPI Research Group developed equations for
estimating GFR fromcreatinine, cystatin C, and the combination
of both, with and without inclusion of a coefficient for Black
race. The concerns about the continued use of race in GFR that
led to the removal of the race coefficient are described in the
rationale that follows Practice Point 1.2.4.2. The 2009 CKD-EPI
creatinine equation includes creatinine, age, race, and sex.
238
The
2021 CKD-EPI creatinine equation was refitted without race and
includes creatinine, age, and sex.
147
As a consequence of not
including the Black race coefficient, the 2021 CKD-EPI
creatinine equation leads to a small overestimate of GFR in
non-Black individuals and a small underestimate in Black
individuals. The 2009 CKD-EPI creatinine equation is more
accurate than the 2021 CKD-EPI creatinine equation in the
non-Black race group, as indicated by the percentage of eGFRs
within 30% of mGFR (P
30
), although the change in the level of
accuracy is small compared with the known variability in
mGFR and eGFR, and P
30
remains at the level consistent with
recommended targets as indicated in prior CKD guidelines
(Table 14
83,91,147,148,235,238–243
Section 1.2.2, Figure 12
174
).
1,147
The 2021 CKD-EPI eGFR creatinine-cystatin C equation that
includes both filtration markers but does not include a term
for Black race leads to improved accuracy in both race groups,
with less difference between race groups in all metrics. The
EKFC developed equations for estimating GFR from creatinine
and cystatin C.
91,240
Before implementation in other regions,
the authors recommended that local regions specify
population-specific Q-values for the creatinine-based EKFC
equation, which is the normal level of creatinine in that region.
To make the SCr-based EKFC equation applicable for children,
age-adjusted Q-values were defined. The original EKFC
creatinine equation had a Q-value developed from Belgium
and Sweden but was validated in 7 European studies and is
recommended for use in White Europeans.
240
They have
recently published Q-values for Black Europeans developed
from a cohort of 90 kidney donors in Paris and for Black
Africans developed from 2 cohorts in République
Démocratique de Congo Cote D’Ivoire. The EKFC cystatin C
equation includes only age and cystatin C, that is, it does not
include sex or race. The Q-value for cystatin C was developed
in a White cohort in Uppsala, Sweden. The cystatin C–based
EKFC equation has been validated in White Europeans, Black
Europeans, White Americans, and Black Africans. To increase
accuracy and precision, EKFC recommends averaging
creatinine and cystatin C to obtain an estimate of GFR that
includes both filtration markers. eGFRcr-cys (the average of
the EKFC creatinine and EKFC cystatin C) also provides the
most accurate estimates, consistent with the findings of CKD-
EPI eGFRcr-cys.
In both the CKD-EPI and EKFC external validation data-
sets, there are consistent findings that the eGFRcr-cys provides
improved performance in estimating mGFR compared with
the respective creatinine- or cystatin-only equations. This re-
inforces the recommendation in Section 1.2.1 emphasizing the
greater use of eGFRcr-cys for decisions that require GFR.
There have been seve ral modifications to the CKD-EPI
equations for use in individual countries, including China, Japan,
and Pakista n.
83,153,235
We expect country-specificmodifications
of both CKD-EPI and EKFC to continue to be developed. One
recent study in China reported no clinically meaningful
differenc e in the performance of the Asian-modified CKD-EPI
and EKFC equations compared with mGFR.
244
Studies vary in their consistency and precision. Direct
comparisons of available estimating equations in populations
with worldwide applicability are lacking, and so too are
validation studies comparing equations against mGFR in all
populations of interest. The overall certainty of the evidence is
therefore low but where the performance characteristics of
GFR estimating equations in the population of interest are
known, there are data to suppor t the use of one equation over
another for improved accuracy of GFR reporting.
Practice Point 1.2.4.2: Use of race in the computation of
eGFR should be avoided.
Estimating equations for GFR have histor ically incor po-
rated demographic variables of age, sex, and race to explain
variation in serum concentrations of endogenous filtration
markers that are unrelated to GFR, thereby minimizing sys-
tematic errors in subgroups defined by these variables and
systematic differences between groups.
Age, sex, and race variables were included in the 2009
CKD-EPI equation as previous studies indicated higher
average SCr for the same mGFR level in people who are older
versus younger, males versus females, and people who are
Black versus non-Black. Incorporation of these variables
minimized systematic errors in groups and systematic dif-
ferences between groups.
147,245
Similarly, subsequent to the
chapter 1 www.kidney-international.org
S188
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table 14 | Validated GFR estimating equations
Marker
Equation name and
year Age Variables Development populations
Creatinine CKD-EPI 2009
238
$18; modification
CKD-EPI 40 for
pediatric available
Developed using A, S, R but
reported not using the Black
race coefficient, A, S, R (NB)
8254 Black and NB individuals from 10 studies
in the United States and Europe
a
CKiD U25 2021
239
1–25 A, S, height 928 children with CKD in the United States and
Canada
CKD-EPI 2021
147
$18 A, S 8254 Black and NB individuals from 10 studies
in the United States and Europe
a
EKFC 2021
240
2–100 A, S, European Black and NB
specific Q-value; separate
Q-values for Africa vs. Europe
mGFR vs. SCr (11,251 participants in 7 studies
in Europe and 1 study from the United States)
Normal GFR from 5482 participants in 12 studies
of kidney donor candidates (100% Caucasian)
European NB Q from 83,157 laboratory samples
(age 2–40 years) in 3 European hospital clinical
laboratories; European Black Q-value (N ¼ 90
living kidney donors from Paris); African Black
Q-value (N ¼ 470 healthy individuals from
République Démocratique de Congo); All
Q-values developed in cohorts independent
for EKFC development and validation
Lund Malmö Revised
2014
241
A, S 3495 GFR examinations from 2847 adults from
Sweden referred for measurement of GFR
CKD-EPI 2009
Modified for
China 2014
b,242
$18 A, S 589 people with diabetes from the Third Affiliated
Hospital of Sun Yat-sen University, China
CKD-EPI 2009
Modified for
Japan 2016
b,83
$18 A, S 413 hospitalized Japanese people in 80 medical
centers
CKD-EPI 2009
Modified for
Pakistan 2013
b,235
$18 A, S 542 randomly selected low- to middle-income
communities in Karachi and 39 people from
the kidney clinic
Cystatin C CKD-EPI 2012
148
$18 A, S 5352 Black and NB individuals from 13 studies
in the United States and Europe
EKFC 2023
91
18–100 A mGFR vs. SCys (assumed to be the same as
mGFR vs. SCr)
Normal GFR (same as for the SCr equation)
Q from laboratory samples from 227,643 (42%
female) laboratory samples from Uppsala
University Hospital, Sweden
CAPA 2014
243
A, S 4690 individuals within large subpopulations of
children and Asian and Caucasian adults
Creatinine-
cystatin C
CKD-EPI 2012
148
$18 Developed using A, S, R but
reported not using the Black
race coefficient, A, S, R (NB)
5352 Black and NB individuals from 13 studies
in the United States and Europe
CKD-EPI 2021
147
$18 A, S 5352 Black and NB individuals from 13 studies in
the United States and Europe
Average of EKFC cr
and cys
240
$2 A, S, European race specific
Q-value; separate Q-values
for Africa vs. Europe
See above for EKFC creatinine and cystatin C
A, age; CAPA, Caucasian and Asian pediatric and adult subjects; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CKiD, chronic
kidney disease in children; cr, creatinine; cys, cystatin C; EKFC, European Kidney Function Consortium; GFR, glomerular filtration rate; mG FR, measured glomerular filtration
rate; NB, non-Black; Q values, median level of serum creatinine or cystatin C in a given population without chronic kidney disease; R, race; S, sex; SCr, serum creatinine; SCys,
serum cystatin C; U25, under 25 years old.
a
Also included 100 Asians and 353 Hispanic or Native Americans.
b
Modified from CKD-EPI or MDRD; modifications may reflect systematic differences in measurement of creatinine and mGFR as well as population differences in non-GFR
determinants of creatinine.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S189

initial publication, the EKFC equation also includ ed as
separate Q-values, the median SCr concentration for Black
Europeans from Paris and Africans from Cote D’Ivoire and
Democratic Republic of the Congo.
91
Race differs from age and sex, as race (and ethnicity) is dy-
namic, shaped by geographic, cultural, and sociopolitical forces,
and thus the definition can change across geography and over
time.
246,247
Consistent with this, in the past several years,
inclusion of race in GFR estimating equations, along with
other algorithms in medicine, faced increasing scrutiny,
particularly in the United States but also elsewhere in the
world.
248–254
Concerns included, first, race is a social and not a
biological construct, and thus the definition of a race group is
subject to change over time. Second, using a binary variable to
assign race groups ignores social and biological diversity within
and among people with similar racial background groups.
Third, there are differences across countries and regions in
self-reported race and ethnicity, thus leading to uncertainty as
to how to apply the term, and blanket use can lead to error.
Thus, even though the inclusion of an indicator for race
group leads to improved accuracy compared with mGFR in
some studies, these concerns and other considerations led to the
2021 recommendation for it not to be used in the computation
of eGFR in the United States.
255
Other countries have also
recognized that race should not be included in computation
and elected to use the CKD-EPI 2009 age, sex, race–non-
Black, as the population of people who are Black was
sufficiently small to not warrant error for other groups.
236,256
We recognize that specific countries or regions (e.g., Japan
and Thailand) have developed “region-specific” equations.
153
We advocate for modifying equations based on the population
being tested.
Special considerations
Pediatric considerations.
Practice Point 1.2.4.3: Estimate GFR in children using
validated equations that have been developed or validated
in comparable populations.
Examples of validated equations include the CKiD under 25
years old (U25) 2021 eGFRcr equation, the EKFC, and the
CKD-EPI40. The Work Group judged that many healthcare
providers would choose the CKiD U25 2021 eGFRcr equation
given it was derived in a multiracial cohort of child ren with
CKD and has been externally validated in cohorts with reduced
and normal GFR. The performance of the CKiD U25 2021
eGFRcr equation is uncertain in the very young, those with very
low GFR, or in populations outside of Europe and North
America.
257
An alternative height/sex/age/creatinine-based
GFR estimating equation is acceptable if it has been shown to
improve accuracy of GFR estimates in the population of
interest (Table 14
83,91,147,148,235,238–243
). The EKFC equation
has been validated in a large cohort of European children (N
¼ 1254), as well as in adults.
240
Of interest, the EKFC
equation in children is the same as in adults. Thus, both
CKiD U25 and EKFC allow a GFR estimation for children
with CKD without changes in calculated eGFR at the
transition between adolescence and young adulthood. In
children with neurological disorders, muscle wasting, or who
have metabolic disorders and are on a very low–protein diet,
a cystatin C–based equation is likely more appropriate.
1.3 Evaluation of albuminuria
Albuminuria refers to abnormal loss of albumin in the urine
(urine ACR $30 mg/g or $3 mg/mmol). Albumin is one type
of plasma protein found in the urine in normal subjects and
in larger quantity in people with kidney disease. In the KDIGO
2012 Clinical Practice Guideline for the Evaluation and
Management of Chronic Kidney Disease,
1
clinical terminology
was changed to focus on albuminuria rather than proteinuria
as albumin is the principal component of urinary protein in
most kidney diseases. Epidemiologic data demonstrate a strong
relationship between the quantity of urine albumin with both
kidney and CVD risk and observed CVD even at very low
levels, and assays to measure albumin are more precise and
sensitive than assays to measure urine protein. We refer to
albuminuria or urine albumin when discussing general
concepts and will refer either to total protein, albumin, or other
specific proteins when discussing that parameter specifically.
1.3.1 Guidance for physicians and other healthcare providers
Practice Point 1.3.1.1: Use the following measurements for
initial testing of albuminuria (in descending order of prefer-
ence). In all cases, a first void in the morning midstream
sample is preferred in adults and children.
(i) urine ACR, or
(ii) reagent strip urinalysis for albumin and ACR with
automated reading.
If measuring urine protein, use the following measu rements:
(i) urine protein-to-creatinine ratio (PCR),
(ii) reagent strip urinalysis for total protein with auto-
mated reading, or
(iii) reagent strip urinalysis for total protein with
manual reading.
Table 15 | Criteria for equation comparison for comparison of
candidate equations to another (i.e., how to determine
validity)
Criteria Consideration
Systematic error (bias): absolute magnitude
of the absolute value of the median
difference ¼ median (eGFR – mGFR)
Small <5
Moderate 5–10
Large >10
Precision: IQR of the difference between
eGFR and mGFR
Small <10
Moderate 10–20
Large >20
Accuracy: P
30
(percentage of estimates
within 30% of mGFR)
Optimal $90
Acceptable 80–90
Poor <80
eGFR, estimated glomerular filtration rate; IQR, interquartile range; mGFR, measured
glomerular filtration rate.
Units for systematic error (bias) and IQR are ml/min per 1.73 m
2
and for units for P
30
are percentages. Equa tions that have large error (bias) or IQR, or low P
30
have poor
performance.
chapter 1 www.kidney-international.org
S190
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 1.3.1.2: Use more accurate methods when
albuminuria is detected using less accurate methods.
Confirm reagent strip positive albuminuria and/or pro-
teinuria by quantitative laboratory measurement and
express as a ratio to urine creatinine wherever possible
(i.e., quantify the ACR or PCR if initial semiquantitative
tests are positive).
Confirm ACR ‡30 mg/g (‡3 mg/mmol ) on a random
untimed urine with a subsequent first morning void in
the morning midstream urine sample.
Practice Point 1.3.1.3: Understand factors that may affect
interpretation of measurements of urine albumin and
urine creatinine and order con fi rmatory tests as indicated
(Table 16).
The practice point advocating for the use of spot samples
measuring albumin or protein g reatly facilitates its incorpo-
ration into clinical practice by avoiding the need for timed
urine collections. Such spot samples can over- or underesti-
mate urine albumin due to variation in dilution. Use of ACR
or protein-to-creatinine ratio (PCR) in spot urine samples
can decrease this error. ACR is an estimate of total urine al-
bumin loss. The creatinine excretion rate varies substantially
between people. ACR or PCR will overestimate urine albumin
loss in people with low creatinine excretion and will under-
estimate urine albumin or protein loss in people with very
high creatinine excretion.
The decision by prior guideline Work Groups not to have a
sex-specific threshold and to use easy-to-remember values
regardless of units may also lead to some misclassification. On
balance, the current Work Group agrees with this approach
given the continued underutilization of urine albumin in the
assessment of CKD.
It is possible that replacing urinary total protein measure-
ment with albumin measurement may cause nonalbuminuric
(effectively tubular and overproduction) proteinuria to be
missed. The significance of this issue is thought to be low in
adults.
258–261
In health, relatively small amounts of albumin (<30 mg/24
hours) are lost in the urine. Urine albumin measurement
provides a more specific and sensitive measure of chan ges in
glomerular permeability than urinary total protein.
262–264
There is evidence that urinary albumin is a more sensitive test
to enable the detection of glomerular pathology associated
with some other systemic diseases including diabetes, hy-
pertension, and systemic sclerosis.
265–268
Total protein measurement is problematic in urine due to
imprecision and insensitivity at low concentrations—relatively
large increases in urine albumin loss can occur without causing a
significant measurable increase in urinary total protein,
264
large
sample-to-sample variation in the amount and composition of
proteins, high and variable concentrations of non–protein
interfering substances relative to the protein concentration,
and high inorganic ion content. Most laboratories currently
use either turbidimetry or colorimetry
269
to measure total
protein. These methods do not give equal analytical specificity
and sensitivity for all proteins, with a tendency
269–271
to react
more strongly with albumin than with globulin and other
non-albumin proteins,
272–275
and many have significant
interferences causing falsely high results.
275–277
There is no
reference measurement procedure and no standardized
reference material for urinary total protein measurement
(https://jctlm.org/). The variety of methods and calibrants in
use means that there is inevitably significant between-
laboratory variation.
278–280
Studies examining the diagnostic accuracy of tests to
quantify urine albumin and other proteins usually compare
tests with laboratory quantification from 24-hour urine col-
lections. It is generally recognized that a 24-hour sample is the
definitive means of demonstrating the presence of
Table 16 | Factors causing biological variation in urine albumin or urine protein
Factor Falsely elevated ACR or PCR False decrease in ACR or PCR
Variability in urine
albumin or protein
Hematuria Increases albumin and protein in the urine
Menstruation Increases albumin and protein in the urine
Exercise
259
Increases albumin and protein in the urine
Infection
260,261
Symptomatic urinary infection can cause
production of protein from the organism
Nonalbumin proteins Other proteins may be missed by albumin reagent strips
Variability in urinary
creatinine concentration
Biological sex Females have lower urinary creatinine excretion,
therefore higher ACR and PCR
Males have higher urinary creatinine excretion,
therefore lower ACR and PCR
Weight
73,160
Low urinary creatinine excretion consistent with low
weight can cause high ACR or PCR relative to
timed excretion
High urinary creatinine excretion consistent with high weight
can cause low ACR or PCR relative to timed excretion
Changes in creatinine
excretion
Lower urinary creatinine excretion with AKI
or low-protein intake
High urinary creatinine excretion with high-protein intake
or exercise
ACR, albumin-to-creatinine ratio; AKI, acute kidney injury; PCR, protein-to-creatinine ratio.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S191

albuminuria. However, timed samples are often collected with
error. Overnight, first void in the morning , second void in the
morning, or random sample collections are therefore rec-
ommended as first-line tests.
281,282
Because creatinine
excretion in the urine is fairly constant throughout the 24-
hour period, the measurement of ACR (or PCR) allows
correction for variations in urinary concentration.
283,284
ACR is a suitable alternative to timed measurement of urine
albumin loss.
285–290
PCR on random or early morning
untimed samples shows good diagnostic performance and
correlation with 24-hour collection.
281,291–298
We acknowledge that reagent strip devices can have a role
in settings where access to laboratory services may be limited
(see Section 1.4).
Implementation of first morning voids will be difficult to
obtain in most healthcare settings. Nephrology offices could
develop protocols to send people with CKD home with a
urine collection container and instruction on how to obtain a
clean catch, which the person brings back before their next
visit. Alternatively, obtaining blood and urine tests before the
next visit can facilitate first morning voids. However, in the
absence of a first morning void, a random sample may still be
used. Negative findings in people at hig h risk for CKD, for
example, where the ur ine sample is diluted, can be confirmed
with a subsequent first morning void. Positive findings in
people at low risk for CKD, where the ACR level is just above
the threshold where the urine samples are concentrated, can
also be confirmed with a first morning void.
The numeric equivalence of ACR in mg/g (mg/mmol) to
approximately g/d is based on the simple assumption that
creatinine excretion rate (CER) approximates 1 gram/d (10
mmol/d). To better estimate urine albumin in individuals with
creatinine generation that is very different from the average, one
might consider measuring a timed urine collection if the value
would affect clinical decisions. As with assessment of GFR using
measured CrCl, use supervised urine collections. Alternatively,
equations are available that estimate creatinine generation from
prediction equations and then multiply that value by the ACR to
compute an estimated albumin excretion rate (AER) that ac-
commodates the lower or higher level of CER.
299,300
Measurement of urinar y albumin is recommended because
it is relatively standardized and because it is the single most
important protein lost in the urine in most CKDs. Use of
urinary albumin measurement as the preferred test for pro-
teinuria detection will improve the sensitivity, quality, and
consistency of approach to the early detection and manage-
ment of kidney disease.
Commonly used reagent strip devices measuring total
protein are insufficiently sensitive for the reliable detection of
proteinuria, do not adjust for urinary concentration, and are
only semiquantitative. Furthermore, there is no standardization
between manufacturers. The use of such strips should be
discouraged in favor of quantitative laboratory measurements
of albuminuria or proteinuria, or validated point-of-care de-
vices for urine albumin/ACR (Section 1.4). When used, reagent
strip results should be confirmed by laboratory testing.
Although the reference point remains the accurately timed
24-hour specimen, it is widely accepted that this is a difficult
procedure to control effectively and that inaccuracies in urinary
collection may contribute to errors in estimation of albumin
and/or protein losses. In practice, untimed urine samples are a
reasonable first test for ascertainment of albuminuria. A first
morning void sample is preferred because it correlates well with
24-hour albumin and/or protein excretion, has relatively low
intraindividual variability, and is required to exclude the
diagnosis of orthostatic (postural) proteinuria. A random urine
sample is acceptable if no first morning void sample is available.
The concentration of albumin or protein in a urine sample will
be affected by hydration (i.e., how diluted or concentrated a
urine sample is), and reporting the albumin or protein to the
creatinine ratio will correct for urinary concentration and
reduce intraindividual variabilit y.
205,261,301,302
There is biological and analytical variability in urine albu-
min and urine protein loss. There are several biological factors
that affect urine albumin or protein loss, separate from kidney
disease (Table 16).
259
All of these can lead to false detection of
CKD or its progression. Thus, positive tests should be
confirmed, especially in people without risk factors for CKD.
Large changes would be repeated to confirm increasing urine
albumin and urine protein. Chapter 2 discusses the
magnitude of change to be considered a real change given the
known biological and analytical variability.
There is also biological variability in urine creatinine
excretion. Change in creatinine concentration in the urine can
also lead to observed changes in ACR or PCR, independently
of changes in protein loss. In general, urine creatinine mea-
surements are less susceptible to factors that interfere with
SCr assays. If a more accurate quantification of albuminuria
or total proteinuria is required, measure urine albumin or
total protein in a timed collection under supervised condi-
tions as recommended above.
Special considerations
Pediatric considerations.
Practice Point 1.3.1.4: In children, obtain a first morning
urine sample for initial testing of albuminuria and pro-
teinuria (in descending order of preference):
(i) Both urine PCR and urine ACR,
(ii) Reag ent strip urinalysis for total protein and for
albumin with automated reading, or
(iii) Reagent strip urinalysis for total protein and for
albumin with manual readi ng.
Consistent with the KDIGO 2012 Clinical Practice
Guideline for the Evaluation and Management of Chronic
Kidney Diseas e,
1
PCR is adv ised and preferred as initial
screening for children as the majority of children have
underlying developmental abnormalities often referred to as
CAKUT and a much higher proportion of children than
adults have tubular pathology.
303
Testing for ACR may miss
tubular proteinuria. However, testing exclusively for
proteinuria does not allow characterization of the source. If
urine PCR is used, urine ACR should also be measured to
chapter 1 www.kidney-international.org
S192
Kidney International (2024) 105 (Suppl 4S), S117–S314

better characterize proteinuria. Significant albuminuria
generally reflects glomerular damage.
304
Importantly, in the
context of screening for children with diabetes, ACR
remains the standard, in line with adult guidelines.
The same considerations of using first morning samples
(because of orthostat ic proteinuria) and considering tran-
siently increased proteinuria during intercurrent illness or
after exercise apply to children as well as adults. Orthostatic
proteinuria is estimated to affect 2%–5% of adolescents.
305
Age and body size are important for interpreting proteinuria
and albuminuria. In term and preterm neonates, PCR is high
(PCR 1000–3000 mg/g [100–300 mg/mmol]) in the first days
and weeks of life, and is related to glomerular and tubular losses
of protein from immature nephrons, as well as very low creati-
nine from low muscle mass. Recent studies outline proteinuria
ranges for neonates, including for preterm and low-birth-weight
neonates. As the tubules mature, proteinuria slowly declines. In
general, a PCR of <500 mg/g (<50 mg/mmol) (or a 24-hour
protein of <150 mg/m
2
/d) is considered normal for infants aged
6 months to 2 years. For children over 2 years, a first morning
urine PCR of <200 mg/g (<20 mg/mmol) protein, or <150 mg/
m
2
/d, or a first morning urine ACR <30 mg/g (<3mg/mmol)is
usually considered normal.
83,306–309
More comprehensive values
can be found in Pediatric Nephrology.
310
1.3.2 Guidance to clinical laboratories
The following comments are focused on the laboratory
assessment of albuminuria, rather than total proteinuria, given
albumin measurement is the preferred approach to proteinuria
evaluation (Section 1.3.1.) However, some of these practice
points (sample type and storage, reporting as a PCR) would
apply equally to total protein measurement practices.
Practice Point 1.3.2.1: Implement the laboratory reporting
and handling standards outlined in Table 17 to ensu re
accuracy and reliability of the findings when assessing
urine samples.
Practice Point 1.3.2.2: Implementation of an external
quality assessment sc heme/program for urin e albumin and
creatinine, including calculation of the ACR, is a pre ferred
practice for laboratories.
Adoption of the reporting and handling standards for
assessment of urine samples is of paramount importance to
ensure that healthcare providers receive urine ACR reports in
a consistent style and w ith assurance regarding the accuracy
and reliability of the result.
Measurement of urine albumin for the detection of kidney
disease as with any analyte should be with methodology
traceable to international standards using a standard reference
material. This is currently not the case, and results may vary
by greater than 40% between laboratories depen ding on the
methodology used with attendant impact on the interpreta-
tion of reported results.
The type of urine collection and the analytical method
influence result interpretation. Twenty-four-hour urine col-
lections present problems in terms of completeness of
collection, specimen storage, and timing accuracy. Therefore,
the assessment of ACR from a single void is a common and
convenient clinical practice. The ACR accounts for hydration
and has similar diagnostic performance to 24-hour urine
AER. The collection method should remain consistent, pref-
erably using the first morning void specimen.
If specimens are being stored for future analysis, careful
attention must be paid to the storage conditions to avoid
degradation of albumin leading to quantification error. The re-
ported effects of frozen storage on urine albumin are somewhat
inconsistent. Albumin is generally stable in urine stored at 2
C–8
C for 7 days. However, losses of albumin have been reported
when urine is stored frozen at temperatures higher than 80
C.
Precipitates often form when urine is stored refrigerated or
frozen but can be redissolved on warming: samples should be
warmed to room temperature and mixed before analysis.
290
Albumin losses may be affected by factors including period of
storage, sample albumin concentration, and individual
variation.
311
It should be possible to provide refrigerated
storage and process samples for albumin measurement in a
laboratory within 7 days in most healthcare settings.
The internationally accepted laboratory quality standards
are variably met worldwide, and laboratories are at different
levels with respect to quality. However, the Work Group placed
a high value on the accuracy and reliability of quantification of
albuminuria and judged that people with CKD, their healthcare
providers, and policy makers would want laboratories to ach-
ieve these reporting and handling standards.
The direct costs of total protein measurement in urine are
lower than those of urine albumin. However, total protein
measurement lacks sensitivity for the detection of low but
clinically significant levels of albuminuria. For this, and other
reasons discussed in Section 1.3.1, the measurement of ACR is
preferred to that of PCR.
Urine albumin should be measured using immunological
assays capable of specifically and precisely quantifying albu-
min at low concentrations and of producing quantitative re-
sults over the clinically relevant range. The biological
variation of urine albumin exceeds 60%. Target analytical
variation (CV) should be based on an optimal level of <0.25
biological variation, approximately 15%. This is in keeping
with good practice recommendations from the National
Academy of Clinical Biochemistry.
312
Table 17 | Implementation standards to ensure accuracy and
reliability of urine samples
Samples for albumin measurement analyzed fresh or stored at 4
C for
up to 7 days
Samples for albumin measurement should not be stored frozen
at 20
C
Report ACR in untimed urine samples in addition to urine albumin
concentration rather than the concentrations alone
Reporting to 1 decimal place for ACR whether mg/mmol or mg/g
Analytical CV of methods to measure urine albumin should be <15%.
ACR, albumin-to-creatinine ratio; CV, coefficient of variation.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S193
Significant progress has been made in developing a certi-
fied reference material for urine albumin and a reference
measurement procedure.
313,314
However, current
commercially available assays for urine albumin are not
standardized against this reference material. Laboratories
should ensure that they are enrolled and demonstrate
satisfactory performance in, an EQA scheme for urine
albumin, creatinine, and ACR.
Urine albumin (and protein) concentrations in ur ine
should be reported as a ratio to creatinine—ACR (or PCR).
Reporting as a ratio to creatinine corrects for variations in
urinary flow rate and enables reporting on untimed, spot
samples, obviating the need for timed, including 24-hour,
collections, which are prone to collection error and tedious
for people to undertake. Reporting albumin as a ratio to
creatinine reduces the intraindividual variability in albumin-
uria compared with reporting as albumin concentration alone
(mg/mmol or mg/g).
315
To aid clarity in reporting across and within healthcare
systems, and to provide guidance regarding the number of
meaningful digits in a result, a standardized approach should
be used in relation to reporting units of ACR and PCR. ACR
results should be expressed to one decimal place (mg/mmol)
or whole numbers (mg/g). Both enzymatic and Jaffe assays
are generally suitable for the measurement of creatinine in
urine, although high concentrations of glucose can interfere
in Jaffe urine creatinine measurement and produce clinically
meaningful errors in ACR.
1.4 Point-of-care testing
Recommendation 1.4.1: We suggest that point-of-
care testing (POCT) may be used for creatinine and
urine albumin measurement where access to a lab-
oratory is limited or providing a test at the point-of-
care facilitates the clinical pathway (2C).
Practice Point 1.4.1: Whenev er a POCT device is used for
creatinine and urine albumin testing, ensure that the same
preanalytical, analytical, and postanalytical quality criteria
relating to the specimen collection and performance of the
device, including external quality assessment, and the
interpretation of the resul t is used.
Practice Point 1.4.2: Where a POCT device for creatinine
testing is being used, generate an estimate of GFR. Use the
equation consistent with that used within the region.
Practice Point 1.4.3: Where a POCT device is being use d for
albuminuria testing, the capability of also analyzing
creatinine and producing an ACR is important. Assess the
ability of the POCT ACR devices to produce a positive
result in 85% of people with significant albuminuria (ACR
‡30 mg/g or ‡3 mg/mmol), as part of the evaluation and
consideration of using the device.
This recommendation places a high value on the advantages
of POCTs including convenience, elimination of sample trans-
portation to the central laboratory, minimal sample processing,
simple analytic process, minim al sample requirement, and im-
mediate availability of results. It places a lower value on the
limited and heterogen eous data related to their diagnostic
accuracy.
Key information
Balance of benefits and harms.
POCTs for both creatinine
and urine albumin have several potential benefits. POCT may
lead to earlier diagnosis, and as a result, earlier treatment of
CKD. They may also be used to monitor CKD progression,
which enables more timely treatment decisions. The rapid
reporting, low cost, and convenience to people w ith CKD
compared with central laboratory testing are also important
benefits of POCTs. However, its provision can raise challenges
in relation to maintenance of analytical and diagnostic per-
formance, and governance arrangements. In addition, these
tests may be less accurate than laboratory testing, which may
lead to misdiagnosis, misclassification, overtreatment, or
undertreatment. The balance of benefits and harms needs
rigorous ev aluation specific to each clinical situation.
For creatinine, the ERT identified a systematic review from
the National Institute for Health and Care Excellent (NICE)/
National Institute fo r Health Research (NIHR) diagnostic
guideline that evaluated point-of-care creatinine tests to assess
GFR before computed tomography (CT) scanning with
contrast media.
316
The ERT also updated the findings of this
systematic review. The review from NICE/NIHR identified
and qualitatively synthesized data from 54 studies on
diagnostic accuracy: eGFR diagnostic accuracy (n ¼ 12),
SCr diagnostic accuracy (n ¼ 7), and correlation and bias
of POC creatinine tests compared with laboratory-based
tests (n ¼ 50). One study
317
was identified in the update of
the NICE/NIHR review assessing POC creatinine test
compared with laboratory standards in a pediatric
population with malaria in Uganda.
These studies covered 3 types of devices: StatSensor, i-
STAT, and ABL devices. In general, al l 3 devices demons trated
acceptable accuracy at lower levels of eGFR (<30 ml/min per
1.73 m
2
).
316
Results showed that i-STAT and ABL devices may
have higher probabilities of correctly classifying people in the
same eGFR categories as the laboratory reference than
StatSensor devices.
For albumin, the ERT identifi ed a systematic review pub-
lished in 2014, by McTaggart et al.,
318
that evaluated the
diagnostic accuracy of quantitative and semiquantitative
protein or albumin urine dipstick tests compared with
laboratory-based tests among people w ith suspected or
diagnosed CKD. The ERT included relevant studies from
this review and conducted an update.
Sixty-five studies (in 66 articles)
319–344,345–368,369–384
eval-
uated the accuracy of quantitative and semiquantitative pro-
tein or albumin dipstick tests in a general population not on
chapter 1 www.kidney-international.org
S194
Kidney International (2024) 105 (Suppl 4S), S117–S314

KRT or receiving end-of-life care. Studies addressed the
following critical outcomes: measurement bias (n ¼ 1),
analytical variability (n ¼ 5), analytical sensitivity (n ¼ 2),
and analytic specificity (n ¼ 63) (Supplementary Table
S5
336,347,363,372,373,377,382–384
). Specificity ranged from 17.5 to
99.5 when evaluative ACR $30 mg/g ($3 mg/mmol) and
30.0–98.7 when evaluative ACR $300 mg/g ($30 mg/
mmol). For PCR, specificity ranged from 80.8–96.9 when
evaluative PCR >200 mg/g (>20 mg/mmol) and 75.6–95.2
when evaluative PCR >500 mg/g (>50 mg/mol).
The ev idence regarding the performance of POCT for
creatinine and urine albumin is heterogeneous limiting the
determination of overall findings across these critical out-
comes. However, given the cost-effectiveness benefits, avail-
ability of the test in the absence of laboratory studies, and the
acceptable test performance, the Work Group judged that in
specific clinical scenarios, POCT should be used.
Certainty of evidence. The certainty of evidence for POCT
for creatinine testing was rated as low due to consistent
reporting of reference standards across all outcomes, with
some concerns regarding patient selection and flow and
timing and directness of the evidence. The certainty of evi-
dence regarding performance of all POCT for urine albumin
was very low based on the Quality Assessment of Diagnostic
Accuracy Studies (QUADAS-2) assessment of individual
studies due to sparse data, heterogeneous findings , and con-
cerns about patient selection, index tests, and unclear
reporting of the reference standards.
Values and preferences. The recommendation suggested
that the majority of people with CKD who have limited access
to laboratories would choose to use POCT. These tests may
facilitate people with CKD being seen at home or in remote
settings. Many people with CKD will value the immediate
results available with POCT versus waiting for the tests from a
lab. In addition, some people with CKD will place a higher
value on avoiding expensive lab tests that may not be covered
by their insurance, difficult travel to central healthcare facil-
ities, and exposure to infection risk in hosp ital. These people
with CKD may also place a lower value on the potential
inaccuracies associated with POCTs compared with in-center
laboratory testing.
Resource use and costs. For people with CKD, the use of
POCTs may be less expensive than tests conducted in a
clinical laboratory. In areas with limited access to healthcare
and insurance, these tests may be cost saving and increase the
detection for CKD.
385,386
For the healthcare system, some
direct reagent and staff costs of POCT tend to be higher on
a per test basis than those of centralized laboratory testing,
but these costs may be offset by other savings in the clinical
pathway, for example, through more rapid disease detection
or avoidance of hosp ital referral.
Considerations for implementation. POCTs may not be
available everywhere. Support from the local laboratory ser-
vice should be sought to guide the purchase, evaluation,
implementation, governance, and ongoing quality assurance
of POCT. The ability to test creatinine in a person’s home may
have applicability to “virtual ward” settings (hospital at
home).
It is worth noting that for albuminuria testing, the Na-
tional Academy of Clinical Biochemistry has proposed that
devices should have 95% sensitivity for the detection of
albuminuria.
312
This is not always achieved by POCT devices,
especially those that produce semiquantitative results.
318
Rationale
POCT can be carried out in a wide range of settings including
primary care, community clinics, rural communities, and
secondary care supporting timely diagnosis, monitoring, and
treatment. Importantly, in locations where laboratory ser vices
may be limited or nonexistent (e.g., rural and remote com-
munities), the ability to test versus not testing blood and
urine was important. Advantages of POCT include conve-
nience, elimination of sample transportation to the central
laboratory, minimal sample processing because the analysis is
of whole blood/urine, simple analytic process, minimal
sample requirement, and immediate availability of results.
However, these tests may be prone to errors and inaccuracies.
For these reasons, the recommendation suggests the use of
these tests based on the specific clinical need or geographical/
social circumstances.
Use of POCT may facilitate access to earlier diagnosis and,
thus, care and can be implemented in rural and remote lo-
cations. The value of POCT for currently underserved pop-
ulations cannot be overstated and should include the capacity
for generating creatinine-based eGFR equations. The POCT
devices used would ideally measure both blood creatinine and
urine for albumin and creatinine to measure ACR and be
standardized and calibrated with similar rigor as is recom-
mended for laboratory tests.
Special considerations
Pediatric considerations.
The ab ility to use a small sample
volume, fingerprick sample as opp osed to venipuncture, may
have applicability to testing in children.
For research recommendations, please see Chapter
6: Research recommendations.
www.kidney-international.org chapter 1
Kidney International (2024) 105 (Suppl 4S), S117–S314 S195

Chapter 2: Risk assessment in people with CKD
2.1 Overview on monitoring for progression of CKD
based upon GFR and ACR categories
Practice Point 2.1.1: Assess albuminuria in adults, or
albuminuria/proteinuria in children, and GFR at least
annually in people with CKD.
Monitoring CKD through the surveillance of albuminuria
and GFR serves to update staging for progno sis, identify
timing of intervention strategies, and assess the effectiveness
of specific treatments. No clear threshold defines a clinically
relevant change in GFR or albuminuria, as any worsening
could reflect deterioration in kidney health. However, over-
interpretation of small changes in these measures may lead to
unnecessary changes in clinical management that could be
unhelpful or even deleterious. Education for healthcare pro-
viders and people with CKD about the variability of specific
laboratory measurements in kidney disease is important to
facilitate understanding and to mitigate inappropriate
changes in treatment strateg ies due to nonclinically significant
fluctuations in either positive or negative directions.
There is an expected variability in GFR caused by both
biological and analytical factors of the biomarkers used
(Figure 12). We have chosen to consider the 95% CI of test
reproducibility for both eGFR and ACR as an important
factor for determining thresholds for clinical evaluation.
The initial evaluation of an observed change in either eGFR
or ACR should be to repeat the test(s) so as to determine if
the observed change is clinically significant progression of
CKD or is within biological and analy tical variability of the
test.
Special considerations
Pediatric considerations.
Monitoring of children in the per-
ipubescent phase should be undertaken more frequently than
the CKD stage–based recommended frequency of monitoring
as puberty is a period of high risk of progression.
387
Reasons
for this are incompletely understood, but potential
mechanisms include inability of diseased kidneys to undergo
the hypertrophy needed to accompany the rapid somatic
growth that characterizes puberty and the negative effect of
increased levels of sex steroids.
388
A study of over 900
children with CKD due to CAKUT showed a decline that
was >10 times faster in creatinine-based eGFR after the
period of peak growth than before that period.
388
The CKiD
study (including children with CKD of any cause) showed
more rapid declines in both eGFR (creatinine- and cystatin
C–based) and mGFR after the period of peak growth velocity
than before.
387
Frequency of monitoring should be
individualized, and informed by the severity of CKD, stage of
puberty, and observed recent rate of progression.
Practice Point 2.1.2: Assess albuminuria and GFR more
often for individuals at higher risk of CKD progression
when measurement will impact therapeutic decisions.
Previous guidelines have suggested the routine monitoring
of albuminuria and GFR. Prior guidelines have suggested
annual monitoring for those with CKD G1–G2, every 6
months for those with CKD G3, every 3 months for CKD G4,
and every 6 weeks for CKD G5 disease. Given the greater risk
of disease progression, those with higher risk of disease
progression should undergo more frequent monitoring
(Figure 13
29
). More frequent monitoring may be indicated in
people with changing clinical status, intercurrent events, and
after therapeutic interventions to assess response and
adherence and ensure safet y. In addition, progression risk
may vary by the etiology of CKD within a specific stage
based on GFR and albuminuria or proteinuria.
Practice Point 2.1.3: For people with CKD, a change in
eGFR of >20% on a subsequent test exceeds the expected
variability and warrants evaluation.
Within-subject variation in measured and eGFR is well
described (Figure 12). Thus, the ability to distinguish between
biological and analytical versus pathological variation in the
mGFR and eGFR is important for healthcare providers and
people with CKD. Studies show that intraindividual
biological variation in eGFR is similar across eGFR
equations: CKD-EPI-creatinine (5.3% [4.5%–6.4%]), CKD-
EPI-cystatin C (5.3% [4.5%–6.5%]), and CKD-EPI-
creatinine-cystatin C (5.0% [4.3%–6.2%]). The reference
change value (RCV) is defined as the threshold of change
that differs from the individual’s prior value with 95% CI;
in a cohort of people with CKD, eGFRcr and eGFRcys had
RCVs ranging from 14%–20% in the positive and negative
directions. Alt hough attention to progressive loss of eGFR is
important, smaller changes in GFR may not be related to
true changes in kidney health, especially if transient and
require cautious interpretation.
Thresholds for CKD progression used in clinical trials and
epidemiolog ical studies are different than those suggested for
monitoring of people with CKD. In research studies, 30%–
40% declines in GFR have been associated with increased risk
for kidney failure, and treatment effects on these endpoints
have been associated with changes in risk for kidney failure.
Because these are evaluated at the group level, small errors in
individual people w ith CKD are minimized.
chapter 2 www.kidney-international.org
S196
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 2.1.4: Among people with CKD who initiate
hemodynamically active therapies, GFR reductions of
>30% on subsequent testing exceed the expected variability
and warra nt evaluation.
Acute eGFR decline after intensive BP control has been
observed in people with CKD, with reductions of 10% – 20%
being typical within the first 3 months of treatment. These
declines in eGFR are hemodynamically mediated, represent-
ing a response to BP falling below the lower threshold of a
person’s autoregulatory response. For many, this initial
decline in eGFR is transient and will stabilize or resolve over
time, as resetting of the autoregulatory function occurs. Thus,
acute rises in SCr (or dec lines in eGFR) of <20% – 30% are
expected and do not warrant changes in therapeutic agents,
which may be important for cardio- and kidney-protective
effects in the long term. This phenomenon is especially
common when using ACEi/angiotensin II receptor blockers
(ARBs), as they both lower BP and alter arteriolar flow
through the glomeruli, and SGLT2i throug h similar hemo-
dynamic mechanisms.
Post hoc analyses of trials of SGLT2i treatment in people w ith
diabetes, heart failure, and CKD suggested that participants
with >10% initial drop in eGFR have similar eGFR trajectories
and kidney benefits from SGLT2i compared with the
“nondipper” who received SGLT2i, except in unusual cases
when the acute “dip” in eGFR was >30% from baseline.
389,390
These findings were consistent across all subgroups.
A significant drop in eGFR (>30%) while initiating anti-
hypertensive agents, renin-angiotensin system inhibitors
(RASi), mineralocorticoid receptor antagonists (MRA), or
SGLT2i should prompt a review into other causes and war-
rants close monitoring. However, healthcare providers should
avoid the urge to stop these kidney-protective agents,
particularly because these earlier “dips” are typically reversible
and not an indication of drug toxicity.
Practice Point 2.1.5: For albuminuria monitoring of people
with CKD, a doubling of the ACR on a subsequent test
exceeds laboratory variability and warrants evaluation.
Small fluctuations in albuminu ria or proteinur ia levels
may not indicate disease progression. Appreciation of factors
that impact albuminuria and changes in the measure is
important for healthcare providers. Routine surveillance us-
ing ACR or PCR is warranted in higher risk people with CKD,
as changes in urine ACR are associated with kidney failure.
Specifically, in large population studies, a doubling of the
ACR within a 2-year duration is associated with an increase in
the risk of progression to kidney failure by 50%–100%.
391,392
Albuminuria categories
Description and range
GFR categories (ml/min/1.73 m
2
)
Description and range
A1
G1
≥90
G2
60–89
G3a
45–59
G3b
30–44
G4
15–29
G5 <15Kidney failure
Severely decreased
Moderately to
severely decreased
Mildly to
moderately decreased
Mildly decreased
Normal or high
A2 A3
Normal to mildly
increased
Moderately
increased
Severely
increased
<30 mg/g
<3 mg/mmol
Screen
1
Screen
1
Treat
1
Treat
1
Treat
3
Treat
3
Treat
1
Treat
2
Treat
2
Treat
3
Treat
3
Treat
3
Treat*
3
Treat
4+
Treat*
3
Treat
4+
Treat
4+
Treat
4+
30–299 mg/g
3–29 mg/mmol
≥300 mg/g
≥30 mg/mmol
CKD is classified based on:
• Cause (C)
• GFR (G)
• Albuminuria (A)
Low risk (if no other markers of kidney disease, no CKD)
Moderately increased risk
High risk
Very high risk
Figure 13 | Frequency of monitoring glomerular filtration rate (GFR) and albuminuria in people with chronic kidney disease (CKD).
Albuminuria and GFR grid reflects the risk of progression by intensity of coloring (green, yellow, orange, red, and deep red). The numbers in the
boxes are a guide to the frequency of monitoring (number of times per year). Reproduced from de Boer IH, Khunti K, Sadusky T, et al. Diabetes
management in chronic kidney disease: a consensus report by the American Diabetes Association (ADA) and Kidney Disease: Improving Global
Outcomes (KDIGO). Kidney Int. 2022;102:974–989.
29
Copyright ª 2022, International Society of Nephrology, American Diabetes Association, and
KDIGO. Published by Elsevier Inc. and American Diabetes Association. All rights reserved.
www.kidney-international.org chapter 2
Kidney International (2024) 105 (Suppl 4S), S117–S314 S197

However, changes in albuminur ia within an individual have
substantial variability, with large fluctuations expected given
that the 95% CI around repeat ACR testing is
approximately 50%. For this reason, the Work Group has
defined a doubling in albuminuria or more as exceeding the
expected variability and warranting evaluation if replicated
upon repeat testing. Conversely, reductions of the ACR by
up to 50% are also consistent with random fluctuation.
Special considerations
Pediatric considerations.
Increases in albuminuria and
proteinuria are also associated with increased risk of disease
progression in pediatric populations. A number of studies in
pediatric subjects detailed in Table 18
225,228,393–398
highlight
the value of measurement of albuminuria/proteinuria.
Considerations in older adults. Urine ACR in older adult
population may be elevated due to the loss of muscle mass
leading to lower SCr and lower urinary CrCl. In older adults
or people with frailty, the interpretation of urine ACR should
take into consideration age-related changes in muscle mass
and/or sarcopenia.
2.2 Risk prediction in people with CKD
The CKD staging heatmaps reflect RRs for each CKD cate-
gory compared with persons who do not have CKD at a
population level; however, a person’s absolute r isk for each
outcome requires the use of risk prediction equations for the
specific adverse event.
Individual-level risk prediction can inform key clinical de-
cisions, improve the patient-healthcare provider dialogue, and
enable personalized care for persons with CKD.
399
The heatmap
concept introduc ed in the KDIGO 2012 CKD guideline
emphasizes the RR of adverse outcomes by levels of eGFR and
albuminuria in populations, and encourages healthcare
providers to classify those people with CKD as high risk for
kidney, cardiovascular, and other adverse events based on those
2 parameters.
400
The heatmaps also reinforce the importance
to all of using both eGFR and ACR for assessing severity and
prognosis of CKD and are color-coded to indicate those RRs in
populations but do not enable individual risk prediction.
However, the people within a specific “cell” on the grid or
within an eGFR/ACR category have a wide range of absolute
risks for each of the adverse outcomes of interest. An indi-
vidual person’s risk for each outcome is influenced by their
underlying etiology of CKD, demographic characteristics,
comorbid conditions, and other factors including lifestyle,
SES, nutrition, and intercurrent events. Thus, the RRs shown
in the heatmap tables can be crudely interpreted as a multi-
plier superimposed upon the aforementioned other charac-
teristics. There can be substantial variability and overlap, up
to 8000% in the risk of CKD progression or 4000% in the risk
of kidney failure, for 2 people in the same heatmap category
or CKD stage ( Figure 14
401,402
)
402
; therefore, individual risk
prediction using accurate and externally validated r isk
equations is important in the personalization of care and
can be used to inform absolute risk for individual people.
The corollary to individualizing absolute risks versus RRs
is appreciating the absolute versus relative benefits of disease-
modifying therapies. Although the relative benefits of medi-
cations such as SGLT2i may appear similar across subgroups,
the actual benefit on specific outcomes is highest among
people who have the higher absolute risks for that
outcome.
403
Risk prediction equations can be used to better
identify these people and perform better than healthcare
Table 18 | Impact of albuminuria/proteinuria on CKD progression in pediatrics
Study Impact of albuminuria/proteinuria
ESCAPE
228
A 50% reduction of proteinuria within the first 2 months of treatment initiation more than halved the risk of progression of kidney
disease over 5 years.
Gluck et al.
225
In a cohort of over 7 million children, 0.1% had CKD G2 or higher. The relative risk of CKD progression, defined as reaching CKD G5 or
having a 50% decline in eGFR, was doubled for those who had $1þ proteinuria on dipstick without hypertension and was
quadrupled for those with proteinuria and hypertension over a median follow-up of 5 years.
CKiD
393
ACR of >300 mg/g (>30 mg/mmol) was associated with an 84% higher risk of disease progression over a median follow-up of 3 years
compared with an ACR of 30 mg/g (3 mg/mmol). PCR of 630 mg/g (71 mg/mmol) was associated with an 87% higher risk of
disease progression compared with a PCR of 140 mg/g (16 mg/mmol).
4C
study
394,395
Each log higher value of ACR was associated with a 50% higher risk of kidney failure or a 50% decline in eGFR over a median
follow-up of 3 years. A 115% increase in albuminuria was associated with faster disease progression after cessation of RASi in children
with advanced CKD.
ItalKids
396
Significantly slower decline in creatinine clearance in people with baseline PCRs of <200 mg/g (<23 mg/mmol) and 200–900 mg/g
(23–102 mg/mmol) when compared with those with a PCR of >900 mg/g (>102 mg/mmol). This translated to higher rates of kidney
survival over 5 years in the lower proteinuria groups: 97% and 94% vs. 45%.
Indian
cohort
397
CKD progression risk within 2 years was tripled for those with proteinuria >2000 mg/g (226 mg/mmol).
Japanese
cohort
398
Risk of CKD progression was 7 times as high for those with proteinuria >2000 mg/g (>226 mg/mmol) compared with those with
lower proteinuria concentrations after adjustment for CKD stage, hypertension, sex, and age.
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; CKiD, chronic kidney disease in children; eGFR, estimated glomerular filtration rate; ESCAPE, Effect of Strict Blood
Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients; PCR, protein-to-creatinine ratio; RASi, renin-angiotensin-system inhibitors.
chapter 2 www.kidney-international.org
S198
Kidney International (2024) 105 (Suppl 4S), S117–S314

provider subjective estimation of risk.
404
Several risk
prediction tools have been developed specifically for people
with CKD, and when implemented, allow healthcare
providers to more precisely estimate risk for individual
people for specific outcomes, which supports a deeper
personalization of CKD management.
405,406
Besides
improving individual risk prediction, these tools may be
used to more effe ctively use special ized and often scarce,
nephrology resources, ident ify people for earlier use of
disease-modifying therapy, or enable personalized
discussions of overall goals of care. Importantly, some of
the developed prediction models have been externally
validated in multiple populations, have high discrimination
performance (C-statistics >0.8 or higher), and are easily
used via online calculators (Table 19
9,10,407–411
).
Recommendation 2.2.1: In people with CKD G3–G5,
we recom mend using an externally validated risk
equation to estimate the absolute risk of kidney
failure (1A).
This recommendation places a high value on the need and po-
tential ben e fi ts for individual risk prediction to deliver person-
alized care for people with CKD. The recommendation is worded
to encourage healthcare providers, patients, researchers, and
policy makers to go beyond broad categories of RR for popula-
tion and to estimate the absolute risk of outcomes for each in-
dividual. The recommendation also places a high value on
externally validated prediction equations that can be applied in
diverse healthcare settings and the need for implementation
science in laboratory information systems and EMRs to enable
the delivery of risk-based care for people with CKD.
Key information
Balance of benefits and harms.
There is a large body of
evidence to support the use of the validated risk equations to
estimate the absolute risk of kidney failure requiring dialysis
or transplant in people with CKD G3–G5. Risk equations
using routinely collected data have been developed, externally
validated, and implemented in labs, EMRs, and health
systems.
408,412,413
with eGFR <60 ml/min/1.73 m
2
(N=350,232)
Kidney failure replacement therapy risk among patients
ba
Risk of 40% decline in eGFR among patients
with eGFR >15 ml/min/1.73 m
2
(N=1,365,272)
CKD categories substantially overlapping multiple risk ranges Nearly all CKD categories substantially overlap multiple risk ranges
CKD
stage
G3aA1 G3bA1 G4A1 G3aA1G3bA1 G4A1G1A1 G2A1 G1A2 G2A2 G1A3 G2A3G5A1 G3aA2 G3bA2 G3aA2 G3bA2G4A2 G4A2G5A2 G3aA3 G3bA3 G4A3 G3aA3 G3bA3 G4A3G5A3
2-year risk of KFRE (%)
100
90
80
70
60
50
40
30
20
10
0
Risk of eGFR 40% decline (%)
80
70
60
50
40
30
20
10
0
Referral thresholds
20%–40%: dialysis access/transplant
>10%: multidisciplinary care
>2%: nephrology
Thresholds
>10%: maximize therapy
>5%: consider multiple medications
>1%: optimize medications
Figure 14 | (a) Predicted risk of kidney failure and (b) ‡40% decline in estimated glomerular filtration rate (eGFR) by chronic kidney
disease (CKD) eGFR (G1–G5) and albumin-to-creatinine ratio (ACR) (A1–A3) categories in Optum Labs Data Warehouse. The lines show
potential thresholds for clinical decisions. KFRE, Kidney Failure Risk Equation. Reproduced from (a) Chen TK, Hoenig MP, Nitsch D, et al.
Advances in the management of chronic kidney disease. BMJ. 2023;383:074216
401
;(b) Grams M, Sang Y, Ballew S, et al. TH-PO890. Risk
prediction: CKD staging is the beginning, not the end. J Am Soc Nephrol. 2022;33:301.
402
Table 19 | Externally validated risk equations for predicting kidney failure in the general (CKD G3–G5) population
Equation Variable Population
Outcome
(time horizon)
Discrimination
and calibration Usability
KFRE
9,10,407,408
www.kidneyfailurerisk.com
www.ckdpc.org/risk-
models.html
Age, sex, eGFR, ACR (4 variable) þ
calcium, phosphate, bicarbonate,
and albumin (8 variables)
>1 million patients, >100,000
events from more than
30 countries
Treated kidney
failure (2–5 yr)
0.88–0.91/þþ
KPNW
410
Age, sex, eGFR, albuminuria,
systolic BP, antihypertensive use,
diabetes, and diabetes
complications
39,013 patients, 1097 events
from the Kaiser Permanente
Health System (United States)
Kidney failure
(5 yr)
0.95/þþ
Landray et al.
411
Sex, SCr, albuminuria, and
phosphate
595 patients, >190 events from
the CRIB and East Kent cohorts
in the United Kingdom
Kidney failure 0.91/þ –
Z6 score
409
SCr, albumin, cystatin C, urea,
hemoglobin, and ACR
7978 patients, 870 events—developed
in the German CKD study, validated
in 3 additional European cohorts
Kidney failure
(5 yr)
0.89–0.92/þ –
ACR, albumin-to-creatinine ratio; BP, blood pressure; CKD, chronic kidney disease; CRIB, chronic renal impairment in Birmingham; eGFR, estimated glomerular filtration rate;
KFRE, Kidney Failure Risk Equation; KPNW, Kaiser Permanente Northwest; SCr, serum creatinine.
www.kidney-international.org chapter 2
Kidney International (2024) 105 (Suppl 4S), S117–S314 S199

Multiple systematic reviews and quality assessments of risk
prediction equations have been performed in the last 10 years,
with the most recent review published in 2020.
405
This review
included 35 development studies and 17 external validation
studies, and described the variables included in the
prediction models and provided a decision aid for selecting
the best model for the prediction horizon and the
underlying etiology of kidney disease. More recently, an
additional externally validated model using serum cystatin
C has also been developed in Germany and externally
validated in 3 European cohorts.
409
A summary of
externally validated models for kidney failure is provided
below and in Table 19.
9,10,407–411
We highlight here 3 validated models, The Kidney Failure
Risk Equation (KFRE), the Veterans Affairs model, and the Z6
Score model. All of these use routinely collected data from
labs or EMRs and have been validated in different pop-
ulations, both in North America and internationally to
varying deg rees. Detailed review of all existing prediction
models is beyond the scope of this documen t.
The KFRE was developed and initially validated in 8391 adults
from 2 Canadian provinces, and subsequently validated in
721,357 individuals from more than 30 countries spanning 4
continents.
9,10
In this large validation study , cohorts from both
general populations and nephrology clinic settings were
included. Discrimination was excellent (C-statistic >0.80 in 28/
30 cohorts), and the use of a calibration factor improved
calibration for some regions outside of North America; the
validation populations now exce ed 2 million individuals in
more than 60 cohorts from nearly every continent.
407,408
The
KFRE is consistently highly accurate and has not been improved
by the addition of longitudinal slopes or variability of eGFR and
urine ACR, or by adding cardiovascul ar comorbidities.
407
A further 2 externally validated models from large US
health systems (Kaiser Permanente North West and Veterans
Affairs) also use routinely collected data and predict kidney
failure with high accuracy within a 5-year horizon.
410,414
Only
1 exte rnally validated model for kidney failure has been
developed using serum cystatin C (Z6 model), and although
it is highly accurate in 4 European cohorts, it has not been
validated in other continents.
409
The Work Group judged that the published externally vali-
dated mod els (delineated in Table 19
9,10,407–411
) all had
sufficient accuracy to be used in clinical settings. Given the
potential benefits and utility of knowing the risk of kidney
failure, patients and healthcare providers should be
encouraged to use these tools. Assessing risk of progression
can aid in optimizing healthcare delivery services, facilitate
the earlier identification of individuals for disease-modifying
therapy, help with planning for modality educatio n, and
identify goals of care planning. There are limited but
supportive studies describing the better prediction of
outcomes when using risk equations compared with care that
is delivered according to isolated eGFR values and clinical
judgment. Potential harms from the use of prediction
equations could result from inappropriate use in the settings
of AKI or AKD or in younger individuals with CKD G1–G2
who may be at high risk of progression but low risk of kidney
failure in the next 5 years. In these people, more proximal
outcomes such as 40% decline in GFR or lifetime r isk were
judged to be more appropriate (i.e., establishing a validated
risk equation for the appropriate outcome of interest, derived
from the population of interest). As described above,
healthcare providers should be cognizant of the impact of
biological and analytical variability in albuminuria and eGFR
values and the subsequent impact on calculation of predicted
risk of kidney failure.
Certainty of the evidence. To assess the certainty of evidence,
the ERTexamined 2 existing systematic reviews addressing the
question of the ability of risk prediction models to predict
kidney failure (see Supplementary Table S6).
406,415
The 2021
review from NICE in the United Kingdom (UK) assessed the
certainty of evidence for a variety of risk-based equations to
predict kidney failure and concluded that there was high-
quality evidence to state that the chosen risk prediction
equations accurately predict kidney failure.
415
There was high
certainty of the evidence (C-statistics were high, and the CIs
were narrow). The Tangri 2013 review did not assess the
certainty of evidence as part of the review (Supplementary
Tables S6–S9
9,85,89,94,96,408,416
).
406
The Work Group ag reed with the NICE assessment and
considered evidence from other systematic reviews and
recently published validation studies. The certainty of evi-
dence was based on the established and growing evidence base
for clinical validation and clinical utility as well as feasibility
for validated risk prediction equations that predict kidney
failure.
Values and preferences. The Work Group judged that the
accurate prediction of kidney failure was of importance to
people with CKD, their families, and healthcare providers,
and that most people with CKD would choose to receive
prognostic information about their individual risk of kidney
failure as part of routine care. For a global guideline, the Work
Group focused on prediction equations that were externally
validated, had a low risk of bias, and included variables that
were routinely available in most healthcare settings.
Resource use and costs. Most externally validated risk
equations for predicting kidney failure use routinely collected
data including laboratory variables suc h as eGFR, albumin-
uria, and serum albumin, phosphate, calcium, or hemoglo-
bin, or info rmation on demographics and comorbid
conditions that can be easily obtained. As such, these models
can be easily implemented at low cost to health systems. Only
1 externally validated model (Z6 Score) used cystatin C, and
its usability in global health will depend on the potential
increased routine availability of cystatin C in laboratories
worldwide.
Considerations for implementation. Given the potential
value of risk prediction models for planning and care de-
cisions, healthcare providers should consider how to integrate
risk prediction models into clinical practice, either in EMRs,
laboratory information systems, or using other mechanisms
chapter 2 www.kidney-international.org
S200
Kidney International (2024) 105 (Suppl 4S), S117–S314

(mobile apps). These should aid clinical workflow and deci-
sion-making and even patient understanding . Where possible,
laboratories should report the results from a validated r isk
equation specific to the region automatically for indiv iduals
with CKD G3–G5 when the required variables are available.
Simpler equations can be implemented and reported when
minimal data are available and more complex equations,
requiring additional variables, can be implemented if the
required data are present.
The reporting of risk in the laboratory reports and EMRs
should be standardized with appropriate guidance on risk
thresholds, when available. Local validation studies can be
performed to determine optimal calibration of the specific
risk prediction equations before implementation. Imple-
mentation of risk equations that are externally validated and
use routinely collected data should be prioritized for health
equity and global health considerations.
Rationale
Risk prediction equations that are externally validated, and
locally calibrated, when possible, can lead to improvement in
the delivery of CKD care. These equations should be used as
they can further personalize care plans for people with CKD
and enable discussions about the benefits and harms of dis-
ease-modifying therapy.
This is a strong recommendation, as the Work Group
judged that the evidence supporting both the clinical validity
and clinical utili ty of risk prediction equations was sufficiently
strong to recommend widespread adoption. The Work Group
judged that most externally validated equations rely on
routinely collected data and could therefore be implemented
equally in low-resource settings. The Work Group also jud ged
that the majorit y of physicians will be comfortable in calcu-
lating the risk of kidney failure and discussing the risk and
related treatment decisions with patients and caregivers.
Special considerations
Pediatric considerations.
Work from the CKiD group (2015)
provides a risk calculator for disease progression, using age, sex,
glomerular versus nonglomerular disease, eGFR, hypertension
and laboratory parameters (calculator available at https://www .
kidney.org/professionals/kdoqi/gfr_calculatorPedRiskCalc).
417
Further analyses combining the CKiD data with that from the
ESCAPE trial (of BP control in CKD progression in children)
resulted in a risk calculator that uses diagnosis, eGFR and
proteinuria, and can be accessed at www.ckdprognosis.
com.
418
The 4-value KFRE has been validated in the CKiD
cohort with good discrimination.
416
However, further
evaluation of the calibration in the cohort revealed
incongruence between predicted and observed outcomes in
those with higher predicted risks of kidney failure (who had
lower observed risks).
419
Considerations regarding sex and gender. There is uncer-
tainty around whether sex assigned at birth or gender identity
is to be used in risk equations. At present, a holistic approach
should be used that takes into account sex assigned at birth,
sex hormone milieu, and gender identity with shared deci-
sion-making with the person with CKD.
Practice Point 2.2.1: A 5-year kidney failure risk of 3%–5%
can be used to determine need for nephrology referral in
addition to criteria based on eGFR or urine ACR, and other
clinical considerations.
In most developing and developed countries, there are
insufficient nephrology care resources to manage al l people
with CKD. Using an objective tool to appropriately triage
those most likely to benefit from referral may help to manage
those nephrology resources in an evidence-informed manner.
Because only a small fraction of the CKD population is at
high risk for progression to kidney failure, those people with
lower risks of progression to kidney failure may be effectively
managed in primary care settings with guideline-based
treatments to delay CKD progression (Figure 15). Referral
criteria for nephrology services that include a risk threshold
of 3%–5% over 5 years have been examined retrospectively
and have also been implemented prospectively in several
healthcare settings.
420,421
In settings within Canada and the UK, retrospective
studies have found that the use of these risk thresholds has
avoided harms from nonreferral or delayed referral of those
progressing to kidney failure.
412
In addition, prospective
evaluation has demonstrated a reduction in nephrology
eGFR-based criteria
Risk-based criteria
Transition from
primary care to
nephrology care
Transition from
nephrology care to
interprofessional care
Access and
transplant
planning
eGFR
90 60 30 20 ≤10
Kidney
failure
KF risk ≥3%–5%
5 years
KF risk ≥10%
2 years
KF risk ≥40%
2 years
eGFR 30–60 eGFR <30 eGFR <20
Figure 15 | Transition from an estimated glomerular filtration rate (eGFR)-based to a risk-based approach to chronic kidney disease
care. KF, kidney failure.
www.kidney-international.org chapter 2
Kidney International (2024) 105 (Suppl 4S), S117–S314 S201

referral wait times, particularly for high-risk individuals. In
other clinical settings with relatively scarce access to
nephrology care, these thresholds should be adjusted to
ensure that wait times are acceptable for local standards.
421
Discussion of risk should also consider the individual
person, their comorbidities, and their risk of death from
other causes.
Practice Point 2.2.2: A 2-year kidney failure risk of > 10%
can be used to determine the timing of multidisciplinary
care in addition to eGFR-based criteria and other clinical
considerations.
People with CKD G4–G5 are more likely to develop con-
current complications of CKD including anemia, hyper-
kalemia, bone mineral disorders, and/or metabolic acidosis
and protein-energy wasting. In addition, they remain at high
risk for adverse events including AKI, emergency department
visits, and hospitalizations. As such, in many countries and
healthcare settings, these people may be enrolled in inter-
disciplinary care clinics or receive care management resources
to reduce morbidity and healthcare costs, and to avoid un-
planned dialysis initiation.
A risk threshold risk of >10% over 2 years has been
studied and implemented in some jurisdictions in Canada as
the key eligibility criteria for access to interdisciplinary care
that includes a nurse, pharmac ist, renal dietitian or accredited
nutrition provider, and other allied health support. This
practice point is based on results from these studies, which
demonstrate acceptance and preference of a risk-based
criteria by patients and providers.
422
Given the costs
associated with delivery of care management resources and
interdisciplinary models, risk-based thresholds offer a useful
guide to the selection of the ideal target patient population
to derive most benefit from the highly specialized team. It
is important to note that people with CKD at earlier stages
or those at lower risk of progression may benefit from an
individual allied health resource (e.g., pharmacist or
dietitian); however, risk-based thresholds provide a guide to
identify people with CKD who benefit most from an entire
multidisciplinary team.
Practice Point 2.2.3: A 2-year kidney failure risk threshold
of >40% can be used to determine the modality education,
timing of preparation for kidney replacement therapy
(KRT) including vascular access planning or referral for
transplantation, in addition to eGFR-based criteria and
other clinical considerations.
The appropriate timing for modality education, timing of
vascular access planning, or referral for transplan tation in a
person with low or declining GFR can be difficult to predict.
Vascular access planning in all adults with CKD G4 would
lead to the unnecessary placement of fistulae, whereas waiting
until eGFR falls below 15 ml/min per 1.73 m
2
may lead to
inappropriate overuse of central venous catheters at dialysis
initiation. Studies have described the potential utility of risk-
based thresholds in planning for dialysis access specifically
and found acceptable specificit y and positive predictive values
for the risk-based threshold criteria as compared with eGFR
alone. The Work Group noted that the KDOQI vascular ac-
cess guideline (2019) currently recommend a risk-based
threshold >50% or eGFR <15 ml/min per 1.73 m
2
for
initiation of vascular access planning, while acknowledging
that access to surgeons and primary failure to maturation
rates may vary by patient and by center.
423
Based on current evidence, a threshold of >40% risk or an
eGFR of 15 ml/min per 1.73 m
2
is acceptable to use for
initiating vascular access referral. Lower risk thresholds, such
as >20%, can optimize sensitivity, can be used to initiate
modality education, and may be appropriate for presurgical
vascular access planning or referral for transplantation in
centers with longer wait times.
Practice Point 2.2.4: Note that risk prediction equations
developed for use in people with CKD G3–G5, may not be
valid for use in those with CKD G1–G2.
The Work Group recognizes that the progression of CKD
can occur at all severities, and that in earlier stages of disease
(G1–G3), large declines in eGFR can occur in 2- to 5-year
time frames without reaching kidney failure (Figure 16
424
).
Risk prediction models developed in populations w ith later
stages of CKD are not accurate in CKD G1–G2, whereas
alternative, accurate, externally validated risk prediction
equations have been developed for predicting 40% decline in
eGFR or kidney failure at all stages of CKD. For this
Patient prole:
50-year-old male with diabetes, eGFR 80 ml/min per 1.73 m
2
, urine ACR 1 g/g
Kidney failure risk: 0.07% over 2 years, 0.23% over 5 years
CKD progression risk: 10.4% over 3 years
0
2
4
6
8
10
12
Risk (%)
2 years 3 years
Kidney failure*
0.07%
CKD progression*
10.4%
Figure 16 | Comparison of risk of chronic kidney disease (CKD)
progression (5-year probability of estimated glomerular filtration
rate [eGFR] <60 ml/min per 1.73 m
2
) versus kidney failure in
adults with CKD G1–G2 calculated from the risk equation
available at https://www.ckdpc.org/risk-models.html . *Kidney
failure risk calculated from Kidney Failure Risk Equation (KFRE). ACR,
albumin-to-creatinine ratio. CKD progression risk from Grams ME,
Brunskill NJ, Ballew SH, et al. Development and validation of
prediction models of adverse kidney outcomes in the population with
and without diabetes. Diabetes Care. 2022;45:2055–2063.
424
chapter 2 www.kidney-international.org
S202
Kidney International (2024) 105 (Suppl 4S), S117–S314
intermediate CKD progression outcome, 3 recent publica-
tions present models for people with or without diabetes,
using both regression and machine learning–based methods,
with or without biomarkers ( Table 20).
8,424,425
Given the
potential utility of these new models to identify high-risk
people for early intervention, they should be used to predict
disease progression in people with CKD G1–G2 and may
supplement established risk equations among people with
CKD G3. People with CKD identified as intermediate risk
(e.g., >1% per year) with these tools may benefit from the
earlier initiation of therapy and closer follow-up, and those
identified as high risk (e.g., >5% per year) may have the
largest benefit from multidrug therapy to slow progression.
Practice Point 2.2.5: Use disease-specific, externally vali-
dated prediction equations in people with immunoglobulin
A nephropathy (IgAN) and autosomal dominant polycystic
kidney disease (ADPKD).
Risk prediction models for specific etiologies of CKD have
also been developed, are externally validated, and used in
healthcare settings to guide clinical care. For autosomal domi-
nant polycystic kidney disease (ADPKD), 2 equations can be
useful in determining the longer-term risk of kidney failure and
may guide therapy with tolvaptan—the Mayo Clinic Classifi-
cation tool and the Predicting Renal Outcome in Polycystic
Kidney Disease (PROPKD) score,
426,427
which incorporates
genetic data. Of these, the Mayo Clinic Classification tool has
been shown to be accurate in external validation.
In people with IgAN, 2 externally validated prediction
tools (clinical or clinical þ histology) have been developed
using large international cohort studies. Models that included
the mesangial hypercellularity (M), endocapillary hyper-
cellularity, segmental g lomerulosclerosis (S), and tubular at-
rophy/interstitial fibrosis (T) (MEST) histological score were
more accurate (C-statistic: 0.81–0.82 vs. 0.78) and showed
improved reclassification in development and external vali-
dation datasets.
428,429
Given the availability of accurate
externally validated models, thes e should be preferentially
used over more general CKD models in people with an
established diagnosis of IgAN or ADPKD. It is important to
note that the clinical presentation of IgAN can include
rapidly progressive disease, and people with rapidly
progressive GN may not have been well represented in the
cohorts use d to develop existing prediction tools.
2.3 Prediction of cardiovascular risk in people with
CKD
Practice Point 2.3.1: For cardiovascular risk prediction to
guide preventive therapies in people with CKD, use externally
validated models that are either developed within CKD
populations or that incorporate eGFR and albuminuria.
Cardiovascular morbidity and mortality disproportion-
ately affect people with CKD, and risk prediction tools
developed in the general (non-CKD) population may un-
derestimate the risk of atherosclerotic CVD (ASCVD) or
heart failure in CKD populations. Absolute risk is used to
determine eligibility for disease-modifying pharmacological
therapy in CVD guidelines, and underestimation of risk may
lead to suboptimal treatment of people with CKD, perpetu-
ating biases (“renalism”) that have existed for more than 2
decades. New models that have been developed specifically in
adults with CKD (QRISK3
430
) and severe CKD (ckdpc.org),
6
and modifications to existing CVD models (pooled cohort
equations [PCE]/Systematic COronary Risk Evaluation
[SCORE]
431
) that include eGFR and albuminuria should be
used to predict cardiovascular events in individuals with
CKD.
390–393,427,428,430,431,432
In the case of the PCE, the
CKD patch significantly improves the calibration of ASCVD
risk, and the eGFR patch improves the prediction of CVD
mortality using SCORE. Recently, the American Heart
Association Predicting Risk of CVD EVENTs (PREVENT
,
pending
) equations were developed in over 6 million US
adults aged 30–79 years without known CVD with
outcomes of incident ASCVD and HF (combined and
separately).
433,434
These equations included eGFR in the
primary model and included ACR in an add-on model, so
they may be particularly appropriate for people with CKD.
Practice Point 2.3.2: For mortality risk prediction to guide
discussions about goals of care, use externally validated
models that predict all-cause mortality specifically devel-
oped in the CKD population.
People with CKD are at high risk of all-cause mor tality,
and the competing risk of death can affect clinical decision-
making, particularly for older adults with CKD G4, who may
simultaneously be at high risk of kidney failure requiring
dialysis. All-cause mortality can be challenging to predict due
to the multiple biological pat hways and differences in
Table 20 | Externally validated risk models for predicting a 40% decline in GFR
Variables Population/events
Time
horizon (yr)
Discrimination
and calibration
CKD-PC
424
16 variables including demography, CVD
risk factors, clinical and laboratory variable
1.6 million adults with or at
risk for CKD
5 0.74–0.77
Klinrisk
8
20 laboratory variables derived from CBC,
chemistry panel, and urine
177,196 adults with CKD G1–G4
or at risk for CKD
1–5 0.84–0.88
KidneyIntelx
425
3 proprietary biomarkers, 5 additional
clinical variables including albuminuria, BP
1146 adults with CKD G1–G3
and diabetes
5 0.77
BP, blood pressure; CBC, complete blood count; CKD, chronic kidney disease; CKD-PC, Chronic Kidney Disease Prognosis Consortium; GFR, glomerular filtration rate.
www.kidney-international.org chapter 2
Kidney International (2024) 105 (Suppl 4S), S117–S314 S203

personal preferences and goals of care that are not captured
by risk prediction models. Models developed by the CKD-PC
for multiple outcomes in CKD G4þ predict the risk of death,
nonfatal CVD event, or kidney failure in adults at 2 and 4
years and w ere developed using multinational data.
6,434
A 5-year mortality model was also developed in the Car-
diovascular Health Study in older adults from the United
States, where the majority of people had CKD G3.
434
Both
models have modest discrimination (C-statistics
approximately 0.70). These may be more appropriate to
identify high-risk groups, where earlier discussions about
conservative care pathways or alternative goals of care may
have been helpful. These models should not be used to
determine the futility of initiating KRT.
For research recommendations, please see Chapter
6: Research recommendations.
chapter 2 www.kidney-international.org
S204
Kidney International (2024) 105 (Suppl 4S), S117–S314

Chapter 3: Delaying CKD progression and managing
its complications
3.1 CKD treatment and risk modification
Practice Point 3.1.1: Treat people with CKD with a
comprehensive treatment strategy to reduce risks of
progression of CKD and its associated complications
(Figure 17 ).
Risk factors associated with CKD progression, CVD, and
other CKD complications are highly interrelated,
435
and
hence so is their management. We use the term “CKD
treatment and risk modification” to encompass the aim of
CKD treatment, which is to impart meaningful beneficial
effects on “CKD manifestations” and on “CKD outcomes”
(Figure 17). CKD manifestations include symptoms and
clinical/laboratory abnormalities associated with CKD,
which confer health implications. These include increased
BP, anemia, dyslipidemia, CKD-mineral and bone disorder
(CKD-MBD), potassium disorders, severe acidosis,
decreased fertility, and increased risk of complications of
pregnancy. CKD outcomes refer to progression to kidney
failure and CKD-associated morbidity and mortality. These
are wide ranging and include several CVDs, hospitalization,
infections, and gout. Reducing the risk of CKD progression
by targeting its underlying pathophysiology may have
beneficial effects on a range of CKD manifestations and
CKD-associated outcomes, although some complications
may need specific targeted interventions. Healthcare systems
should aim to provide safe and proven cost-effective
therapies that achieve CKD treatment and risk modification
and to minimize limitations to access for people with CKD
as their disease can substantially impact on QoL and
healthcare system resources. A key goal for healthcare
providers should be to identif y people at risk and to start
such treatments early in the course of CKD to maximize
potential benefits.
This chapter provides evidence-based guidelines to sup-
port holis tic management of the risks associated with CKD
(Figure 18). Previously published KDIGO clinical practice
guidelines for the management of BP, diabetes, lipids,
anemia, and CKD-MBD in CKD are available and support
our statements.
19–21,23,436
This chapter also describes certain
laboratory abnormalities including bicarbonate, potassium,
and uric acid, together with a summary of the observed
ranges associated with different stages of CKD
3.2 Lifestyle factors
Practice Point 3.2.1: Encourage people with CKD to under-
take physical activity compatible with cardiovascular health,
tolerance, and level of frailty; achieve an optimal body mass
index (BMI); and not to use tobacco products. Referral to
providers and programs (e.g., psychologists, renal dietitians
or accredited nutrition providers, pharmacists, physical and
occupational therapy, and smoking cessation programs)
should be offered where indicated and available.
Impact on CKD pathophysiology
CKD manifestations
Prevention and treatment of clinical
symptoms and signs (including blood pressure)
Maximize health-related quality of life, physical function,
capacity to work, and ability to socialize
Appropriate monitoring and treatment of laboratory
abnormalities of CKD associated with implications for
health (e.g., anemia, CKD-MBD, potassium disorders, acidosis)
•
•
•
CKD outcomes
Minimize risk of progression to kidney failure
Manage risk and appropriate treatment of
complications, including cardiovascular diseases,
hospitalization, gout, infections, etc.
•
•
Modication of the natural course
of CKD and its symptoms
Figure 17 | Chronic kidney disease (CKD) treatment and risk modification. CKD-MBD, chronic kidney disease-mineral and bone disorders.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S205

This practice point calls out the need for a comprehensive
and integrated approach to lifestyle modification and recog-
nizes that in some circumstances, there is value in referring
people to professionals or programs with expertise in lifestyle
modification. We also appreciate that different healthcare
systems and regions will have variable access to such
specialized services or teams, and thus availability may be an
issue.
3.2.1 Avoiding use of tobacco products
The Work Group concurs with the previous KDIGO recom-
mendations to advise people with diabetes and CKD who use
tobaccotoquitusingtobaccoproducts
23
and extends that advice
to all people with CKD who use tobacco products to reduce the
risk of associated premature mortality from CVD, as well as risk
of respiratory diseases and cancer.
437
Intensive nurse-led
programs appear effective at supporting smoking abstinence
and can be combined with pharmacological intervention (e.g.,
nicotine replacement therapy of nicotine-receptor partial
agonists) to improve smoking abstinence over 16 weeks.
438
See
the KDIGO 2021 Clinical Practice Guideline for the
Management of Blood Pressure in Chronic Kidney Disease
21
and KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease
23
for full details.
Healthy diet Weight management
Stop use of
tobacco products
Physical activity
SGLT2i
continue until dialysis
or transplant
Aim for SBP <120 mm Hg
RAS inhibitor* at maximum
tolerated dose (if HTN)
Statin-based therapy
moderate- or
high-intensity statin
Manage hyperglycemia
as per the KDIGO
Diabetes Guideline,
including use of
GLP-1 RA where indicated
Use ns-MRA in
people with diabetes
and an indication
for use
Dihydropyridine CCB
and/or diuretic if
needed to achieve
individualized
BP target
Antiplatelet
agent for
clinical ASCVD
ASCVD risk, lipids
BP
Lifestyle
First-line
drug therapy for
most patients
+
Targeted therapies
for complications
Steroidal MRA if needed
for resistant hypertension
if eGFR ≥45
Ezetimibe, PCSK9i
indicated based on
ASCVD risk and lipids
Manage anemia,
CKD-MBD, acidosis,
and potassium
abnormalities,
where indicated
Use the same principles
to diagnose and manage
ASCVD and atrial brillation
as in people without CKD
Regular
risk factor
reassessment
(every 3–6
months)
Figure 18 | Holistic approach to chronic kidney disease (CKD) treatment and risk modification. *Angiotensin-converting enzyme inhibitor
or angiotensin II receptor blocker should be first-line therapy for blood pressure (BP) control when albuminuria is present; otherwise
dihydropyridine calcium channel blocker (CCB) or diuretic can also be considered. All 3 classes are often needed to attain BP targets. Icons
presented indicate the following benefits: blood pressure cuff ¼ blood pressure–lowering; glucometer ¼ glucose-lowering; heart ¼ heart
protection; kidney ¼ kidney protection; scale ¼ weight management. ASCVD, atherosclerotic cardiovascular disease; CKD-MBD, chronic kidney
disease-mineral and bone disorder; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon-like peptide-1 receptor agonist; HTN,
hypertension; KDIGO, Kidney Disease: Improving Global Outcomes; MRA, mineralocorticoid receptor antagonist; ns-MRA, nonsteroidal
mineralocorticoid receptor antagonist; PCSK9i, proprotein convertase subtilisin/kexin type 9 inhibitor; RAS, renin-angiotensin system; SBP,
systolic blood pressure; SGLT2i, sodium-glucose cotransporter-2 inhibitor. Modified from Kidney Disease: Improving Global Outcomes Diabetes
Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1–S127.
23
Copyright ª 2022, KDIGO: Kidney Disease Improving Global Outcomes. Published by Elsevier Inc. on behalf of the International Society of
Nephrology. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
chapter 3 www.kidney-international.org
S206
Kidney International (2024) 105 (Suppl 4S), S117–S314

3.2.2 Physical activity and optimum weight
The Work Group concurs with all the recommendation and
practice points relating to physical activity from the KDIGO
2022 Clinical Practice Guideline for Diabetes Management
in Chronic Kidney Disease
23
and considers that they should
extend to all adults with CKD. We draw attention to the
following statements:
Recommendation 3.2.2.1: We recommend that peo-
ple with CKD be advised to undertake moderate-
intensity physical activity for a cumulative duration
of at least 150 minutes per week, or to a level
compatible with their cardiovascular and physical
tolerance (1D).
Practice Point 3.2.2.1: Recommendations for physical ac-
tivity should consider age, ethnic background, presence of
other comorbidities, and access to resources.
Practice Point 3.2.2.2: People with CKD should be advised
to avoid sedentary behavior .
Practice Point 3.2.2.3: For people at higher risk of falls,
healthcare providers should provide advice on the intensity
of physical activity (low, moderate, or vigorous) and the
type of exercises (aerobic vs. resistance, or both).
Practice Point 3.2.2.4: Physicians should consider advising/
encouraging people with obesity and CKD to lose weight.
BMI relates to levels of adiposity on a population scale (though
imperfectly), and a BMI of ov er 25 kg/m
2
in adults (i.e., overweight
or obese) is associated with an increased risk of multiple chronic
diseases including development of CKD .
439,440
Such adiposity-
CKD associations appear to be causal.
441,442
BMI can
over estimate risk in people with high muscle mass,
443
and risk
for a given BMI may vary by ethnicity (with Asians being at
higher risk of metabolic disorders at lower BMIs than
Europeans).
443,444
Nev ertheless, it is important to pr o vide people
with CKD advice about their weight using BMI in conjunction
with other information, including ethnicity, diet, comorbidity ,
physical activity levels, risk of falls, and laboratory values.
Special considerations
Pediatric considerations.
Practice Point 3.2.2.5: Encourage chi ldren with CKD to
undertake physical activity aiming for World Health Or-
ganization (WHO)–advised levels (i.e., ‡60 minutes daily)
and to achieve a healthy weight.
The WHO recommends 60 minutes of moderate-to-vigorous
physical activity daily for children 5–17 years old, including aer -
obic activities as well as activities that strengthen muscle and
bone.
445
Limits on sedentary time, particularly screen time, are
also recommended. For childr en 1–5yearsofage,180minutes
per day of physical activity is recommended; young children in
thisagegroupshouldnotberestrained(i.e.,inastrolleror
carrier) for >60 minutes at a time. Only 13.4% of 224
participants of the CKiD study aged $12 years (median: 15
years) met these WHO targets,
240,25 7,303,30 5,446
compared with
25% of general population children of comparable age.
447
Less
than 2% of CKiD participants met screen time
recommendations (<2 hours per day on school days)
compared with 27% of the general population. Physical activity
has numerous benefits for cardiovascular, mental, and social
health. Given that children with CKD are at higher risk for
problems in all these areas, physical activity may be even more
important in the CKD population.
3.3 Diet
Practice Point 3.3.1: Advise people with CKD to adopt
healthy and diverse diets with a higher consumption of
plant-based foods compared to animal-based foods and a
lower consumption of ultraprocessed foods.
Practice Point 3.3.2: Use renal dietitians or accredited
nutrition providers to educate people with CKD about di-
etary adaptations regarding sodium, phosphorus, potas-
sium, and protein intake, tailored to their individual needs,
and severity of CKD and other comorbid conditions.
Plant-based diets use proportionately more plant-based food
choices, and animal-based food is consumed in moderation.
Diets such as Dietary Approaches to Stop Hypertension
(DASH) are rich in fruits, vegetables, whole grains, and low-fat
dairy foods. A Mediterranean diet pattern is built around
vegetables, fruits, herbs, nuts, beans, whole grains, and seafood
but also includes moderate amounts of dairy, meat, and eggs.
By definition, vegan and vegetarian diets are plant-based. A
whole-food, plant-based diet low in animal-based and ultra-
processed foods may be helpful to slow the progression of CKD
and delay need for dialysis via reduction of cardiometabolic risk
factors such as hypertension, CVD, diabetes, and obesity.
448,449
Ultraprocessed foods such as sugar-sweetened beverages, fast
foods, frozen meals, chips, candy, and pastries are high in
salt, sugar, and fat, and low in nutritional value, and they
promote inflammation, which may contribute to worsening
kidney function. A plant-based diet is rich in anti-
inflammatory nutrients, fiber , and phytochemicals, and has
been shown to reduce proteinuria and decrease metabolic
acidosis.
448,449
The probiotic nature of plant-based foods may
also support the microbiome and reduce inflammation and
intestinal production of uremic toxins.
450
A recent systematic
review evaluated the association of dietary patterns and
kidney-related outcomes.
451,452
Dietary patterns that include
more plant-based unprocessed protein have been
demonstrated, in cohort studies and small RCTs, to slow the
trajectory of eGFR decline, reduce the risk of kidney failure,
reduce risk of mortality, and improve scores in some QoL
domains (e.g., DASH and Mediterranean diet).
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S207

3.3.1 Protein intake
Recommendation 3.3.1.1: We suggest maintaining a
protein intake of 0.8 g/kg body weight/d in adults
with CKD G3–G5 (2C).
Practice Point 3.3.1.1: Avoid high protein intake (>1.3 g/kg
body weight/d) in adults with CKD at risk of progression.
Practice Point 3.3.1.2: In adults with CKD who are willing
and able, and who are at risk of kidney failure, consider
prescribing, under close supervision, a very low–protein
diet (0.3–0.4 g/kg body weight/d) supplemented with
essential amino acids or ketoacid analogs (up to 0.6 g/kg
body weight/d).
Practice Point 3.3.1.3: Do not prescribe low- or very low–
protein diets in metabolically unstable people with CKD.
This recommendation places a higher value on slowing the
rate of GFR decline without the challenges associated with
adherence to lower-protein diets, potential adverse effects, and
the contraindications in people with sarcopenia, cachexia, or
undernutrition. The Work Group judged that many well-
informed people with CKD G3–G5 would choose to implement
this recommendation.
Key information
Balance of benefits and harms.
The Work Group considered
that maintaining a protein intake of 0.8 g/kg body weight per
day in adults in the absence of indications for a higher or
lower protein intake was congruent with a person’s culture
and QoL. Consid erations for protein restriction in the context
of individual preferences, true impact on CKD progression
based on etiology, and other factors need to be considered
and weighed against any potential adverse impacts, such as
malnutrition.
In many societies, most adults and older adults consume
more protein than recommended, with average protein intakes
of 1.2 g/kg/d.
453,454
There is general agreement that, in the
absence of intercurrent disease, the protein requirements for
people with CKD are not different from those of healthy
subjects.
455
The Work Group thus suggests maintaining a
protein intake of 0.8 g/kg body weight/d, a target consistent
with the WHO Recommended Dietary Allowances for the
general population.
456,457
Figure 19
23
shows some examples
of the amount of protein in grams that would be
recommended based on body weight. Clinicians should
advise people with CKD not to confuse grams of protein per
day with the weight of food in grams (i.e., 100 g of meat
contains only approximately 25 g of protein; Figure 20
23
).
Unlike carbohydrates and fats, excess dietary proteins
cannot be stored in the body and are catabolized, leading to
accumulation of protein waste products such as urea and
other uremic toxins. As CKD progresses, these byproducts
accumulate and affect organ function. High-protein intake
also contributes to increased intraglomerular pressure and
glomerular hyperfiltration, which, in turn, may lead to glo-
merulosclerosis and tubulointerstitial injury.
458,459
Progressive decline in kidn ey function is also associated
with a spontaneous loss of appetite potentially leading to
inadequate protein and ener gy intake.
460
The Work Group
therefore encourages maintaining protein intake in adults
with CKD within the recommended range around 0.8 g/kg
body weight/d, and particularly avoiding excess protein
intakes (>1.3 g/kg of body weight/d), which may be
harmful for the kidney.
455
There is observational evidence
suggesting that excess protein intake may accelerate kidney
functional decline.
461–463
The protein type, not only the quantity, may also be
relevant. Table 21 briefly summarizes the impact of plant-
based diets in people with CKD.
464–470
In another cohort
study of older subjects (N ¼ 291, mean age 76 years) with
eGFR <60 ml/min per 1.73 m
2
, there was no significant
association between vegetable protein intake and change in
eGFR.
460
Observational studies
452,464,465
and an RCT
466
have associated a higher plant-based protein relative to
animal-based protein consumption or adherence to plant-
based protein dominant diets with slower eGFR decline
over time and lower risk of death; no study so far has
assessed the measures of patient preferences.
471
It is unclear
whether the associations are attributed to plant-based
protein intake per se or to other nutrients or lifestyle habits
that accompany the plant-based protein intervention;
however, there is biological plausibility. A crossover study of
10 healthy individuals fed for 3 weeks evaluated the effect
of a plant-based protein diet versus an animal-based protein
diet on kidney function parameters. Both diets provided the
same amount of total protein per day. Compared with
animal-based protein, a plant-based protein diet reduced
renal plasma flow, increased renal vascular resistance, and
lowered the fractional clearance of albumin.
472,473
Body weight (kg)
Grams of protein per
day (wt × 0.8 g/kg)
35
28
40
32
50
40
55
44
60
48
65
52
70
56
75
60
80
64
85
68
90
72
95
76
100
80
Figure 19 | Protein guideline for adults with chronic kidney disease not treated with dialysis. wt, body weight in kg. Reproduced from
Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic
Kidney Disease. Kidney Int. 2022;102:S1–S127.
23
Copyright ª 2022, KDIGO: Kidney Disease Improving Global Outcomes. Published by Elsevier
Inc. on behalf of the International Society of Nephrology. This is an open access article under the CC BY-NC-ND license (http://creativecommons.
org/licenses/by-nc-nd/4.0/).
chapter 3 www.kidney-international.org
S208
Kidney International (2024) 105 (Suppl 4S), S117–S314

A low-protein intake (<0.8 g/kg body weight/d) reduces
uremia and uremic toxin generation and improves kidney
hemodynamics by constricting the glomerular afferent arte-
rioles and lowering intraglomerular pressure.
458,474,475
Low-
protein diets and very low–protein diets have been used for
almost a century as a strategy to reduce clinical symptoms
and postpone the need to start maintenance dialysis
treatment. They may also reduce uremic complications and
symptoms, such as metabolic acidosis and phosphate load.
476
The Work Group considers that the evidence does not sup-
port a recommendation to follow low-protein diets alone (i.e.,
0.4–0.6 g/kg of body weight/d) as a strategy to slow the pro-
gression of CKD. In a meta-analysis of people with CKD without
diabetes,
477
a low-protein diet compared with a normal-protein
diet in participants with CKD G3a and G3b (9 studies) or CKD
G4 (1 study) found little or no difference in mortality or eGFR
decline and little or no difference in the number of
participants who reached kidney failure (6 studies, 1814
participants: RR, 1.05; 95% CI: 0.73–1.53). Similar null
associations were observed in a meta-analysis of 8 studies
involving people with diabetes and CKD.
478
However, there is some evidence that very low–protein diets
(i.e., 0.3–0.4 g/kg of body weight/d) under strict supervision
can favorably impact kidney outcomes. A meta-analysis of
studies including people with CKD G4–G5 without diabetes
reported that very low–protein diets compared with low-pro-
tein diet or no rmal-protein diet may reduce the number of
people who reach kidney failure (10 studies, 1010 participants:
RR, 0.65; 95% CI: 0.49– 0.85).
477
Data on adverse effects, such
as weight loss and malnutrition, and on QoL were limited.
There is limited evidence on the use of very low–protein diets
in peopl e with CKD and diabetes.
23
Animal proteins
Meat, poultry, sh, seafood, eggs:
28 g (1 oz) = 6–8 g protein
1 egg = 6–8 g protein
Dairy, milk, yogurt, cheese: 250
ml (8 oz) = 8–10 g protein
28 g (1 oz) cheese = 6–8 g protein
Plant proteins
Legumes, dried beans, nuts, seeds:
100 g (0.5 cup) cooked = 7–10 g protein
Whole grains, cereals:
100 g (0.5 cup) cooked = 3–6 g protein
Starchy vegetables, breads:
2–4 g protein
Figure 20 | Average protein content of foods in grams. Reproduced from Kidney Disease: Improving Global Outcomes Diabetes Work Group.
KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1 –S127.
23
Copyright ª 2022,
KDIGO: Kidney Disease Improving Global Outcomes. Published by Elsevier Inc. on behalf of the International Society of Nephrology. This is an
open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Table 21 | Impact of plant-based foods in people with CKD
Study (N); study design CKD stage or GFR Intervention (follow-up) Outcome
CRIC
467
(N ¼ 2403);
observational
20–70 ml/min per 1.73 m
2
High DASH vs. low DASH (14 yr) CKD progression: HR: 0.83; 95% CI: 0.69–0.99
Mortality: HR: 0.75; 95% CI: 0.62–0.90
NHANES
468
(N ¼ 1110);
observational
30–59 ml/min per 1.73 m
2
DASH by quintiles (7.8 yr) Kidney failure relative hazard (RH) compared
with quintile 5: quintile 1: RH: 1.7; 95% CI: 1.1–2.7;
quintile 2: RH: 2.2; 95% CI: 1.1–4.1
CORDIOPREV
466
(N ¼ 53); RCT
<60 ml/min per 1.73 m
2
Mediterranean diet vs.
low-fat diet (5 yr)
Decline in GFR 3.72 ml/min per 1.73 m
2
vs. 5.4 ml/min per 1.73 m
2
, P ¼ 0.03
CKD QLD
469
(N ¼ 145);
observational
CKD G3 –G4 High vegetable and nut intake
(median 36 mo)
Composite all-cause mortality, kidney failure,
or doubling of SCr: HR: 0.61, 95% CI: 0.39–0.94
REGARDS
470
(N ¼ 3972);
observational
<60 ml/min per 1.73 m
2
Plant-based diet (6 yr) All-cause mortality: HR: 0.77; 95% CI: 0.61–0.97
NHANES III
465
(N ¼ 5346);
observational
<60 ml/min per 1.73 m
2
Increasing plant-to-protein
ratio (8.4 yr)
All-cause mortality for every 33% increase:
HR: 0.77, 95% CI: 0.61–0.96
Longitudinal study of aging
women
464
(N ¼ 1374);
observational
Baseline 65.6 13.1 ml/min
per 1.73 m
2
Higher vs. lower intake of
plant-based protein (10 yr)
Each 10 g higher intake of plant-based protein
reduced a decline in GFR by 0.12 ml/min
per 1.73 m
2
per year
CI, confidence interval; CKD, chronic kidney disease; CKD QLD, Chronic Kidney Disease in Queensland; CORDIOPREV, CORonary Diet Intervention with Olive oil and car-
diovascular PREVention study; CRIC, Chronic Renal Insufficiency Cohort; DASH, Dietary Approaches to Stop Hypertension; GFR, glomerular filtration rate; HR, hazard ratio;
NHANES, National Health and Nutrition Examination Survey; RCT, randomized controlled trial; REGARDS, Reasons for Geographic and Racial Differences in Stroke; SCr, serum
creatinine.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S209

Very low–protein diets are usually vegetarian or vegan diets
and may risk deficiencies of some essential amino acids. A
strategy to counteract this is the addition of supplements of
essential amino acids or precursors of essential amino acids (i.e.,
ketoacid analogs). By lacking the amino group, ketoacid analogs
serve as substrates for protein synthesis without the production
of toxic nitrogenous waste products. Limitations of ketoacid
analogs are, however, high pill burden and high cost. Fifteen
months of strict adherence to vegetarian very low–protein diets
of 0.3 g/kg body weight/d supplemented with 0.125 g/kg body
weight/d of ketoacids in people with an average eGFR of 18 ml/
min per 1.73 m
2
at baseline slowed the decline in eGFR,
increased bicarbonate levels, and reduced the need for KRT
compared with a control group that ate a mixed-protein diet of
0.6 g/kg body weight/d.
479
A recent pragmatic trial was not able
to reproduce this finding,
480
potentially attributed by the
authors to challenges in patient compliance with the treatment.
There is concern that low-protein and very low–protein diets
may result in malnutrition. However, in the meta-analysis by
Hahn et al.,
477
12 studies reported no evidence of malnutrition
in their study participants, whereas 3 studies reported small
numbers of participants in each arm with worsening
nutritional status. The estimated average requirement for
protein intake in adults is approximately 0.5–0.6 g/kg body
weight/d, which corresponds to the amount of protein
required to avoid negative nitrogen balance.
456
Thus under
correct supervision, these diets may not lead to malnutrition.
Malnutrition may arise if the reduction in protein is followed
by a reduction in energy intake. This may be preventable by
adequate patient education on food choices and close
supervision by renal dietitians or accredited nutrition
specialists. A patient-centered approach involves a shared
understanding of treatment goals, effective communication to
alleviate anxieties around food or food misconceptions,
individualized advice that matches cultural values and
preferences, and assistance with implementation of dietetic
advice in the face of a large symptom burden.
Some of the trials on low-protein diets were conducted
before treatment with RASi was introduced, and all of them
before the SGLT2i era. Because the mechanism of action of
these medicat ions and that of low-protein and very low–
protein diets are complementary, it has been postulated that
these strategies may synergize and maximize their combined
effect on delaying CKD progression.
475,481,482
Studies are
needed to demonstrate this hypothesis.
Low- or very low–protein diets are not indicated in people
who are metabolically unstable or during periods of metabolic
instability. This includes conditions that may exacerbate the
risk of malnutrition in the context of low-protein intake, such
as sarcopenia, cachexia, active inflammatory or infectious
diseases, per iods of hospitalization or the early postoperative
period, poorly controlled diabetes, consumptive diseases such
as cancer, treatment of antibiotic or immunosuppressive
medications, and significant short-term loss of body weight.
Certainty of evidence. The certainty of evidence was moder-
ate that there was little to no difference in the critical outcome of
all-cause death and kidney failure prevention when comparing
very low–protein to low- or normal-protein diets, and moderate
that there was some benefit to the critical outcome of kidney
failure for the comparison of very low–protein diets with low- or
normal-protein diets as demonstrated by the wide CIs for these
outcomes including potential for important benefits and harms.
In addition, there was important and unexplained heterogeneity
present. It is uncertain whether low- or very low–protein diets
impact a change in GFR.
The certainty of evidence was very low when comparing low-
protein to normal-protein diets for a change in GFR and low when
comparing very low–protein to low- or normal-protein diets. This
is because the CIs included potential for important benefits and
harms. There was important and unexplained heterogeneity
present; the outcome was reported as a surrogate outcome; and
there was unclear allocation c oncealment in 4 studies.
The overall certainty of evidence for the remaining out-
comes was very low because of increased risk of bias and
small studies with wide CIs. In addition, many studies were
unclear about allocation concealment/random sequence
generation, and had significant, unexplained heterogeneity,
wide CIs for important benefits and harms, and use of sur-
rogate outcomes.
Values and preferences. The Work Group judged that some
clinically suitable people would choose to implement a diet
with protein of 0.8 g/kg body weight/d unless there are
conditions that contraindicate such as sarcopenia, cachexia,
or undernutrition. In addition, the Work Group judged that
protein restriction would be implemented by many people as
a way of managing their kidney disease. It will also have an
impact on overall QoL with the adoption of a more plant-
based diet; however, there may be challenges with imple-
menting and adhering to these changes.
Resource use and costs. The risks, benefits, resource use,
and costs of dietary protein interventions should be consid-
ered when treating people with CKD. The Work Group
considered that plant-based proteins could have a cost-benefit
effect compared with animal-based protein, but evidence in
this topic remains limited.
Considerations for implementation. Protein restriction
without support and advice from renal dietitians or other
accredited nutrition providers may result in low dietary di-
versity and limited food choices, adversely impacting QoL
and altering fundamental components of a person’s culture
and daily life. The use of culturally appropriate foods that are
more familiar to people, nutritional status, goals of care, QoL,
and patie nt preferences should be considered in the imple-
mentation of these recommendations and practice points.
Rationale
The Work Group suggests dietary protein interventions based
on consideration of the possible benefits of plant-based foods,
kidney protection, and avoidance of adverse effect of unsu-
per vised protein restriction. People with CKD not on dialysis
with or without diabetes may opt for some degree of dietary
protein moderation, especially as control of dietary intake
chapter 3 www.kidney-international.org
S210
Kidney International (2024) 105 (Suppl 4S), S117–S314

empowers people with CKD and supports self-care manage-
ment. People put a large value on diet, cultural preferences,
and QoL; however, adherence to a low-protein diet remains
challenging, may impact social and psychological well-being,
and given that most of the trials for protein restriction were
conducted before RASi and SGLT2i were implemented, may
not be worth the sacrifice/change in lifestyle. The impact of
protein restriction and the use of non–animal-based protein
diets should be evaluated in the context of new care para-
digms to ascertain the incremental gain of these strategies
relative to the efforts and costs.
Special considerations
Pediatric considerations.
Practice Point 3.3.1.4: Do not restrict protein intake in
children with CKD due to the risk of growth impairment.
The target protein and energy intake in children with CKD
G2–G5 should be at the upper end of the normal range for
healthy children to promot e optimal growth.
Children with CKD likely have similar resting energy
expenditure to healthy children and should have total energy
requirements in the normal range.
483
As in adults, protein
restriction was considered for children with CKD in the past.
Two RCTs have compared low-protein versus normal-protein
diets in children with CKD.
484,485
One found poorer growth
for those on a low-protein diet, and the other found no
difference in eGFR between the groups. A 2007 Cochrane
meta-analysis concluded that there was uncertainty over the
possible harm of strict low-protein diets on growth in young
infants.
486
The 2009 KDOQI guidelines and the 2020 Pediatric
Renal Nutrition Taskforce suggest maintaining an intake of
dietary protein at 100%–140% of the dietary reference intake
(DRI) or the SDI for ideal body weight in children with CKD
G3 and at 100%–120% of the DRI/SDI in children with CKD
G4–G5.
487,488
Older adults.
Practice Point 3.3.1.5: In older adults with underlying
conditions such as frailty and sarcopenia, consider higher
protein and calorie dietary targets.
In older adults with CKD, nutritional management should
consider potential challenges stemming from simultaneous
and potentially conflicting risks of CKD progression and
malnutrition/protein-energy wasting . In older adults, protein
targets should be set after careful individual assessment to
identify the most urgent clinical challenge.
Geriatric guidelines recommend protein intakes of 1.0–1.2 g/
kg body weight/d to prevent age-related malnutrition and pre-
vent sarcopenia.
489
Such protein intakes may be appropriate in
some people with stable or slowly progressing CKD, whose
clinical picture is dominated by old age and related challenges
to their nutritional and functional status. On the other hand,
protein restriction may be appropriate in older adults whose
primary clinical challenge is CKD with significant progression,
provided they are metabolically stable.
490
The course of action
should consider patient preferences and when necessary ,
involve family members and caregivers.
3.3.2 Sodium intake
The Work Group concurs with the following recommenda-
tion from the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease
23
and the
KDIGO 2021 Clinical Practice Guideline for the
Management of Blood Pressure in Chronic Kidney Disease.
21
Recommendation 3.3.2.1: We suggest that sodium
intake be <2 g of sodium per day (or <90 mmol of
sodium per day, or <5 g of sodium chloride per day)
in people with CKD (2C).
Practice Point 3.3.2.1: Dietary sodium restriction is usually
not appropriate for patients with sodium-wasting
nephropathy.
Global average sodium intake is estimated to be 4310 mg/
d (10.78 g of salt per day), which far exceeds the physiological
requirement and is more than double the WHO recommenda-
tion of <2 g of sodium (equivalent to <5 g of salt) per day in
adults.
491
This perhaps reflects the pervasive use of sodium in
many commercial food products, which makes achieving
WHO targets challenging to meet for many people. There are
large-scale RCTs quantifying the benefits of restricted salt
intake (e.g., using 75% sodium and 25% potassium chloride
salt substitutes) to lower BP and reduce the risk of
cardiovascular events in the general population.
492
In RCTs
with up to 36 weeks of follow-up, reduction in dietary sodium
has also been shown to lower BP and levels of albuminuria in
people with CKD.
492–494
Although presumed to reduce the risk
of CKD progression and CVD, longer term trials have not
been conducted to confirm these effects translate into reduced
riskofclinicaloutcomesinCKD.
493
Given the effects of
sodium restriction on BP, it is reasonable to recommend
sodium restriction to people with CKD in combination with
pharmacological strategies to minimize the risk of kidney and
CVDs. People with CKD may have salt-wasting kidney disease,
malnutrition, or be exposed to extremely hot climatic
conditions. In such scenarios, this recommendation may not
apply.
Special considerations
Pediatric considerations.
Practice Point 3.3.2.2: Follow age-based Recommended
Daily Intake when counseling about sodium intake for
children with CKD who have systolic and/or diastolic blood
pressure >90th percentile for age, sex, and height.
The WHO recommends that the maximum intake of <2g/d
sodium (<5 g/d salt) in adults should be adjusted downward
based on the energy requirements of children relative to those
of adults (Table 22
495
). Children born with low birth weight
(<2.5 kg) are at increased risk for CKD in later life and may
also be at higher risk for hypertension and increased salt
sensitivity. Salt sensitivity is a physiological trait by which BP
in some people exhibits changes parallel to changes in salt
intake. Children born with low birth weight may have a 37%
increased salt sensitivity (definedasanincreaseinmeanBP
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S211
$3 mm Hg over 24 hours while on a high salt diet, when
compare d with a controlled salt diet). That sensitivity may
increase further in those who are small for gestational age.
496
Children with CKD often have underlying tubular condi-
tions that predispose them to numerous electrolyte losses,
including sodium. For these children, a supplemented rather
than restricted sodium intake will be required. For non–salt-
wasting children, salt intake should be limited to the age-
based Recommended Daily Intake.
3.4 Blood pressure control
The Work Group concurs with the KDIGO 2021 Clinical
Practice Guideline for the Management of Blood Pressure in
Chronic Kidney Disease, which encourages individualized BP
targets and use of agents according to age, coexistent CVD, and
other comorbidities; risk of progression of CKD; and tolerance
to treatments.
21
We highlight the following guidance:
Recommendation 3.4.1: We suggest that adults
with high BP and CKD be treate d with a target
systolic blood pressure (SBP) of <120 mm Hg,
when tolerated, using standardized office BP
measurement (2B).
Practice Point 3.4.1: Consider less intensive BP-low ering
therapy in people with frailty, high risk of falls and frac-
tures, very limited life expectancy, or symptomatic postural
hypotension.
RCTs have not demonstrated that intensive BP-lowering
results in meaningful reductions in the risk of kidney failure,
but the RCT evidence supporting important cardiovascular
benefits should encourage such a strategy. By aiming for an SBP
<120 mm Hg, more adults with CKD will achieve an SBP
<130 mm Hg, even if they do not meet the <120 mm Hg
target.Observationally,onaverage,each20mmHghigherthan
usual SBP and 10 mm Hg higher diastolic BP is associated with
an approximate doubling of cardiovascular risk, with no lower
limit down to at least 115/75 mm Hg.
497
Data from the S ystolic
Blood Pressure Intervention Trial (SPRINT) support the SBP
target of <120 mm Hg (when measured using a standardized
office BP mea sure ment) to r educ e cardiovascular risk in
adults aged >75 years, or aged >50 years with 1 or more of
the following risk factors: clinical or subclinical CVD (other
than stroke), eGFR 20–60 ml/min per 1.73 m
2
,or$15% 10-
year cardiovascular risk.
498
Compared with a target of 140
mm Hg, this approach reduces the risk of major adv erse
cardiovascular events (MACE) by one-quarter (hazard ratio
[HR]:0.75;95%CI:0.64–0.89), and that relativ e benefitwas
similar in people with and without CKD. The SPRINT trial
excluded people with diabetes, but cardiovascular benefits of
intensive BP lowering on risk of stroke and heart failure are
clearly apparent in people with diabetes in individual patient
level data meta-analysis of intensive versus standard BP-
lowering trials.
499
Standardized BP monitoring can be challenging to offer in a
clinic setting due to the time required
500
;however,itis
considered potentially hazardous to apply the recommended
SBP target of <120 mm Hg to BP measurements obtained in a
nonstandardized manner.
500
A practical solution to ensure the
identification of high BP is by using home-based monitoring
(or telemonitoring). Trials have shown that 2 morning and
evening BP measurements taken during the first week of every
month can be used to titrate antihypertensive medication and
reduce BP more than “usual care” approaches.
501
People who are frail, have limited life expectancy, or have a
history of falls and fractures may have increased risk of addi-
tional events if BP targets of <120 are achieved. Postural
hypotension in these people is associated with adverse out-
comes, and thus weighing the benefits of some attenuation of
eGFR decline versus the life-changing impact of falls, fractures,
and other events should be considered in choosing specific
targets.
Special considerations
Pediatric considerations.
The Work Group concurs with the
KDIGO 2021 Clinical Practice Guideline for the Management
of Blood Pressure in Chronic Kidney Disease, and we high-
light the following gui dance
21
:
Recommendation 3.4.2: We suggest that in chi ldren
with CKD, 24-hour mean arterial pressure (MAP) by
ambulatory blood pressure monitoring (ABPM)
should be lowered to £50th percentile for age, sex,
and height (2C).
Practice Point 3.4.2: Monitor BP once a year with ABPM
and every 3–6 months with standardized auscultatory office
BP in children with CKD.
Practice Point 3.4.3: In children with CKD, when ABPM is
not available, it is reasonable to target manual auscultatory
office SBP, obtained in a protocol-driven standardized
setting, of 50th–75th percentile for age, sex, and height
unless achieving this target is limited by signs or symptoms
of hypotension.
These statements with respect to children are generally
worded to maintain consistency with the KDIGO 2021
Clinical Practice Guideline for the Management of Blood
Pressure in Chronic Kidney Disease,
21
where the full rationale
and evidence behind the statements are available. However,
the suggestion to target auscultatory office SBP at the 50th–
75th percentile when ABPM is not available departs from
Table 22 | Age-based sodium intake recommendations
495
Age Recommended adequate sodium intake (g/d)
0–6 mo 0.110
7–12 mo 0.370
1–3 yr 0.370
4–8 yr 1.0
9–13 yr 1.2
14–70 yr 1.5
chapter 3 www.kidney-international.org
S212
Kidney International (2024) 105 (Suppl 4S), S117–S314

the BP guideline (the previous guideline suggested a target
<90th percentile). Although office BP may be higher than
BP measured by ABPM, this is not universally the case.
Given the evidence that intensive BP control may slow CKD
progression together with the very low risk of adverse
effects of intensive BP lowering in children,
228
we consider
that more intensive BP lowering targeting around the 50th
percentile is reasonable. However, a target even lower than
the 50th percentile has not been shown to offer additional
benefits. Recent trial data found that using a target of office
auscultatory SBP at 50th to 75th percentile versus intensive
control to below the 40th percentile did not result in
significant differences in left ventricular mass index.
229
3.5 Glycemic control
Please refer to the KDIGO 2022 Clinical Practice Guideline
for Diabetes Management in Chronic Kidney Disease for
specific recommendations, practice points, and research
recommendations.
3.6 Renin-angiotensin system inhibitors
The Work Group highlights recommendations from the
KDIGO 2021 Clinical Practice Guideline for the Management
of Blood Pressure in Chronic Kidney Disease and selected
practice points for treatment with RASi from the KDIGO 2021
Clinical Practice Guideline for the Management of Blood
Pressure in Chronic Kidney Disease
21
and the KDIGO 2022
Clinical Practice Guideline for Diabetes Management in
Chronic Kidney Disease.
23
The Work Group considers
several recommendations to apply even in the absence of
high BP and has adapted the recommendations from the BP
guideline to remove this requirement. Key recommendations
and practice points are highlighted:
Recommendation 3.6.1: We recommend starting
renin-angiotensin-system inhibitors (RASi) (angio-
tensin-converting enzyme inhib itor [ACEi] or
angiotensin II receptor blocker [ARB]) for people
with CKD and severely increased albumi nuria (G1–
G4, A3) without diabetes (1B).
Recommendation 3.6.2: We suggest starting RASi (ACEi
or ARB) for people with CKD and moderately increased
albuminuria (G1–G4, A2) without diabetes (2C).
Recommendation 3.6.3: We recommend starting
RASi (ACEi or ARB) for people with CKD and
moderately-to-severely increased albuminuria
(G1–G4, A2 and A3) with diabetes (1B).
Recommendation 3.6.4: We recommend avoiding
any combination of ACEi, ARB, and direct renin in-
hibitor (DRI) therapy in people with CKD, with or
without diabetes (1B).
Practice Point 3.6.1: RASi (ACEi or ARB) should be
administered using the highest ap proved dose that is
tolerated to achieve the benefits described becaus e the
proven benefits were achieved in trials using these doses.
Practice Point 3.6.2: Changes in BP, serum creatinine,
and serum potassium should be checked within 2–4
weeks of initiation or increase in the dose of a RASi,
depending on the current GFR and serum potassium.
Practice Point 3.6.3: Hyperkalemia associated with use
of RASi can often be managed by measures to reduce the
serum potassium levels rather than decreasing the dose
or stopping RASi.
Practice Point 3.6.4: Continue ACEi or ARB therapy
unless serum creatinine rises by more than 30% within
4 weeks following initiation of treatment or an increase
in dose.
Practice Point 3.6.5: Consider reducing the dose or dis-
continuing ACEi or ARB in the setting of either symp-
tomatic hypotension or uncontrolled hyperkalemia
despite medical treatment, or to reduce uremic symptoms
while treating kidney failure (estimated glomerular filtra-
tion rate [eGFR] <15 ml/min per 1.73 m
2
).
Practice Point 3.6.6: Consider starting people with CKD
with normal to mildly increased albuminuria (A1) on RASi
(ACEi or ARB) for specific indications (e.g., to treat hyper-
tension or heart failure with low ejection fraction).
The role of RASi (specifically ACEi or ARB) in the manage-
ment of BP and people with CKD, diabetes, and/or high BP have
been specifically considered in recent KDIGO guidelines.
21,23
Although temporarily stopping RASi may be a valid treatment
strategy for emergent hyperkalemia, we advise to ensure the
reinitiation of treatments once the adverse event is resolved, so
that people are not deprived of a needed medication (Practice
Point 4.3.3).
502–506
The Work Group offers the new Practice
Point 3.6.6 and a revised algorithm for initiation of RASi
(Figure 21).
23
The algorithm has been updated to suggest a
$30% decrease in eGFR (rather than increase in creatinine)
should be a trigger to investigate for an underlying other
condition. This represents a threshold above which the eGFR
change is greater than would be expected from natural
variation. Lastly, it should be noted that restricting salt intake
may help ensure maximal effects of RASi.
Practice Point 3.6.7: Continue ACEi or ARB in people
with CKD even when the eGFR falls below 30 ml/min per
1.73 m
2
.
In a recent STOP-ACEi trial of 411 participants with mean
eGFR of 13 ml/min per 1.73 m
2
, a policy of discontinuing
RASi in CKD G4–G5 did not result in any kidney or car-
diovascular benefits.
507
Two observational studies have also
found that associations suggesting outcomes were worse
among participants who stopped RASi after reaching an
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S213

eGFR <30 ml/min per 1.73 m
2
, compared with those who
continue.
508,509
In addition, a recent individual patient level
data meta-analysis demonstrated a benefit in delaying KRT
in patients with eGFR <30 ml/min per 1.73 m
2
.
510
3.7 Sodium-glucose cotransporter-2 inhibitors
(SGLT2i)
The Work Group concurs with the KDIGO 2022 Clinical
Practice Guideline for Diabetes Management in Chronic
Kidney Disease, which stated: “We recommend treating pa-
tients with type 2 diabetes (T2D), CKD, and an eGFR $20 ml/
min per 1.73 m
2
with an SGLT2i (1A).”
23
However, in this
guideline, we offer a more general 1A recommendation for
adults with CKD. We also highlight practice points from
the KDIGO Diabetes guideline for diabetes management in
CKD, which are also relevant for people with CKD without
diabetes:
Recommendation 3.7.1: We recommend treating
patients with type 2 diabetes (T2D), CKD, and an
eGFR ‡20 ml/min per 1.73 m
2
with an SGLT2i (1A).
Practice Point 3.7.1: Once an SGLT2i is initiated, it is
reasonable to continu e an SGLT2i even if the eGFR falls
below 20 ml/min per 1.73 m
2
, unless it is not tolerated
or KRT is initiated.
Practice Point 3.7.2: It is reasonable to withhold SGLT2i
during times of prolonged fasting, surgery, or critical
medical illness (when people may be at greater risk for
ketosis).
Recommendation 3.7.2: We recommend treating
adults with CKD with an SGLT2i for the following
(1A):
eGFR ‡20 ml/min per 1.73 m
2
with urine ACR ‡200
mg/g (‡20 mg/mmol), or
heart failure, irrespective of level of albuminuria.
Practice Point 3.7.3: SGLT2i initi ation or use does not
necessitate alteration of frequency of CKD monitoring and
the reversible decrease in eGFR on initiation is generally
not an indication to discontinue therapy.
Use of SGLT2i in people with T2D is recommended in previous
guidelines irrespective of level of albuminuria. This new recom-
mendation (3.7.2) places high value on the importance of reducing
risk of kidney failure, cardiovascular mortality, and heart failure
in people with CKD and high value on the large relative reductions
in risk for kidney disease progression in a series of large, placebo-
controlled RCTs. It also places moderate value on the benefits of
SGLT2i on risk of AKI, hospitalization for heart failure and
myocardial infarction, risk of hospitalization from any cause,
and high value on the demonstrable net absolute benefits
versus absolute harms in people with CKD (particularly in those
Initiate ACEi or ARB
Monitor serum creatinine and potassium
(within 2–4 weeks after starting or changing dose)
<30% decrease
in eGFR
Increase dose of ACEi or ARB
or continue on maximally
tolerated dose
≥30% decrease
in eGFR
Normokalemia Hyperkalemia
• Review for causes of AKI
• Correct volume depletion
• Reassess concomitant medications
(e.g., diuretics, NSAIDs)
• Consider renal artery stenosis
• Review concurrent drugs
• Moderate dietary potassium
intake
• Consider:
- diuretics
- sodium bicarbonate
- potassium binders
Reduce dose or stop ACEi or ARB
if mitigation strategies ineffective
Figure 21 | Algorithm for monitoring of potassium and estimated glomerular filtration rate (eGFR) after the initiation of renin-
angiotensin system inhibitors. ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB, angiotensin II receptor blocker;
NSAID, nonsteroidal anti-inflammatory drug. Modified from Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022
Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1–S127.
23
Copyright ª 2022, KDIGO:
Kidney Disease Improving Global Outcomes. Published by Elsevier Inc. on behalf of the International Society of Nephrology. This is an open
access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
chapter 3 www.kidney-international.org
S214
Kidney International (2024) 105 (Suppl 4S), S117–S314

without diabetes who are at very low risk of ketoacidosis). SGLT2i
also favorably reduce BP, uric acid levels, measures of fluid over-
load, the risk of serious hyperkalemia, and do not increase risk of
hypoglycemia. The recommendation is consistent with but ex-
pands on Recommendation 1.3.1 from the KDIGO 2022 Clinical
Practice Guideline for Diabetes Management in Chronic Kid-
ney Disease to include people with causes of CKD not related to
diabetes.
Key information
Balance of benefits and harms. Benefits.
Several large, pla-
cebo-controlled RCTs have provided clear demonstrations of
the efficacy of SGLT2i, which substantially reduce the risk of
kidney failure, AKI, and hospitalization for heart failure, and
also moderately reduce the r isk of cardiovascular death and
myocardial infarction in pe ople with and without CKD. These
benefits appear to be irrespective of diabetes status, cause of
kidney disease, or level of GFR.
511,512
The benefits of SGLT2i
in people with diabetes and CKD have been fully described in
the KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease.
23
Two large RCTs using 2 different SGLT2i recruited 10,913
participant s and focused on CKD populations at risk of
progression, reporting benefits in terms of kidney disease
progression.
403,513
Key differences between the 2 trials were
the inclusion of a large number of causes of kidney disease
not related to diabetes, lower eGFR, and lower levels of
ACR in The Study of Heart and Kidney Protection With
Empagliflozin (EMPA-KIDNEY) compared with the
Dapagliflozi n and Prevention of Adverse Outcomes in
Chronic Kidney Disease (DAPA-CKD) trial.
In a collaborative metanalysis including those 2 and 11
other trials (13 trials with just over 90,000 randomized par-
ticipants) in comparison with placebo, those allocated to an
SGLT2i experienced a 37% reduction in the risk of kidney
disease progression and a 23% reduction in the risk of AKI
irrespective of diabetes status (Figure 22).
511
The same meta-analysis showed that, compared with pla-
cebo, allocation to an SGLT2i reduced the risk of the com-
posite of cardiovascular death or hospitalization for heart
failure by 23% irrespective of diabetes status (Figure 23
511
),
although there were limited numbers of cardiovascular
events in people with CKD without diabetes. SGLT2i also
afford an approximate 10% RR reduction in MACE,
primarily from reduced risk of cardiovascular death and
myocardial infarction with no clear effect on stroke.
511,512
Furthermore, SGLT2i al so importantly reduce the risk of
hospitalization from any cause,
403
reduce BP,
403,513,514
uric
Mean
baseline eGFR,
ml/min per
1.73 m
2
Events/participants
SGLT2
inhibitor
Placebo
Event rate per
1000 patient-years
SGLT2
inhibitor
Placebo
Event rate per
1000 patient-years
SGLT2
inhibitor
Placebo
Kidney disease progression
RR
(95% CI)
Events/participants
SGLT2
inhibitor
Placebo
RR
(95% CI)
Acute kidney injury
0.750.25 0.5 1.00 1.50
Favors SGLT2 inhibitor Favors placebo
0.750.25 0.5 1.00 1.50
Favors SGLT2 inhibitor Favors placebo
Diabetes
DECLARE−TIMI 58
CANVAS Program
VERTIS CV
EMPA−REG OUTCOME
DAPA−HF
EMPEROR−REDUCED
EMPEROR−PRESERVED
DELIVER
CREDENCE
SOLOIST−WHF
SCORED
DAPA−CKD
EMPA−KIDNEY
Subtotal: diabetes
No diabetes
DAPA−HF
EMPEROR−REDUCED
DELIVER*
EMPEROR−PRESERVED
DAPA−CKD
EMPA−KIDNEY
Subtotal: no diabetes
Total: overall
85
77
76
74
63
61
60
60
56
51
44
44
36
67
68
63
63
62
42
39
56
65
56/8582
80/5795
49/5499
51/4645
18/1075
13/927
38/1466
33/1578
153/2202
NA/NA
37/5292
103/1455
108/1525
739/40,041
10/1298
5/936
17/1551
12/1531
39/697
119/1779
202/7792
941/47,833
102/8578
81/4347
32/2747
47/2323
24/1064
23/929
44/1472
37/1572
230/2199
NA/NA
52/5292
173/1451
175/1515
1020/33,489
15/1307
10/938
17/1557
18/1519
70/701
157/1790
287/7812
1307/41,301
1.6
3.6
2.6
4.0
12
13
15
9.5
27
..
5.0
35
36
..
5.0
5.2
5.0
4.5
29
35
..
..
3.0
5.8
3.4
7.6
16
24
18
11
41
..
7.0
60
59
..
8.0
10
4.9
6.9
53
47
..
..
0.55 (0.39–0.76)
0.61 (0.45–0.83)
0.76 (0.49–1.19)
0.51 (0.35–0.76)
0.73 (0.39–1.34)
0.52 (0.26–1.03)
0.82 (0.53–1.27)
0.87 (0.54–1.39)
0.64 (0.52–0.79)
..
0.71 (0.46–1.08)
0.57 (0.45–0.73)
0.55 (0.44–0.71)
0.62 (0.56–0.68)
0.67 (0.30–1.49)
0.50 (0.17–1.48)
1.01 (0.51–1.97)
0.68 (0.33–1.40)
0.51 (0.34–0.75)
0.74 (0.59–0.95)
0.69 (0.57–0.82)
0.63 (0.58–0.69)
Trend across trials sorted by eGFR:
Diabetes P=0.87;
No diabetes P=0.86;
Heterogeneity by diabetes status: P=0.31
125/8574
30/5790
42/5493
45/4687
31/1073
26/927
60/1466
59/1578
86/2200
25/605
116/5291
48/1455
73/1525
766/40,664
18/1295
20/936
30/1551
37/1531
16/697
34/1779
155/7789
921/48,453
175/8569
28/4344
22/2745
37/2333
39/1063
33/929
84/1472
52/1572
98/2197
27/611
111/5286
69/1451
81/1515
856/34,087
30/1305
34/938
47/1558
47/1519
21/701
54/1790
233/7811
1089/41,898
3.5
1.6
2.5
2.5
19
21
20
17
17
55
16
15
24
..
9.9
16
8.8
12
11
10
..
..
4.9
2.5
2.7
6.2
24
27
28
15
20
59
16
22
27
..
16
28
14
15
15
16
..
..
0.69 (0.55–0.87)
0.66 (0.39–1.11)
0.95 (0.57–1.59)
0.41 (0.27–0.63)
0.79 (0.50–1.25)
0.77 (0.46–1.28)
0.69 (0.50–0.97)
1.13 (0.78–1.63)
0.85 (0.64–1.13)
0.94 (0.55–1.59)
1.04 (0.81–1.35)
0.66 (0.46–0.96)
0.88 (0.64–1.20)
0.79 (0.72–0.88)
0.60 (0.34–1.08)
0.56 (0.32–0.98)
0.64 (0.41–1.02)
0.80 (0.52–1.23)
0.75 (0.39–1.43)
0.63 (0.41–0.97)
0.66 (0.54–0.81)
0.77 (0.70–0.84)
Trend across trials sorted by eGFR:
Diabetes P=0.02;
No diabetes P=0.66;
Heterogeneity by diabetes status: P=0.12
Figure 22 | Effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) with kidney disease outcomes by diabetes status. ACR, albumin-
to-creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; NA, not applicable; RR, relative risk. Reproduced from Nuffield
Department of Population Health Renal Studies Group, SGLT Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the
effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet.
2022;400:1788–1801.
511
ª 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S215

acid levels,
515
weight/fluid overload,
516
and the risk of serious
hyperkalemia.
517
Harms. SGLT2i are well tolerated with high levels of
adherence in the RCTs in CKD.
403,513,514
In the studied
populations, any risk of ketoacidosis or lower-limb
amputation resulting from SGLT2i use was substantially
lower than the potential absolute benefits and generally
restricted to people with diabetes. Meta-analysis estimates of
absolute benefits and harms for each 1000 people with
CKD and T2D treated for 1 year with an SGLT2i were 11
fewer cardiovascular deaths or hospitalizations for heart
failure, for approximately 1 episode of ketoacidosis and
approximately 1 lower-limb amputation, respectively (and
also 11 fewer peop le developing kidney disease progression
and 4 fewer people with AKI). The corresponding benefits
in peop le w ith CKD without diabetes were 15 fewer people
with kidney disease progression, 5 fewer with AKI, and 2
fewer cardiovascular deaths or hospitalizations for heart
failure per 1000 patient-years of treatment with no excess
risk of ketoacidosis or amputation observed.
511
The vast
majority of urinary tract infections in people taking SGLT2i
are not caused by SGLT2 inhibition, and there is no
increased risk of hypoglycemia. There is an increased r isk of
mycotic genital infections (in men and women), but these
are generally mild and treating these infections with low-
cost topical agents should help treatment adherence.
Certainty of evidence. SGLT2i have been studi ed in a
series of large tr ials with consistent effects obser ved be-
tween trials, u sing different agents in the class. The tr ials
have robust double-blind designs that minimize risk of
bias, and the y have provided precise estimates of effect
with no risk of publication bias due to the Nuffield
Department of Public Health (NDPH) Renal Stud ies
Group and SGLT2 in hibitor Meta-A nalysis Cardio-Renal
Trial ists’ Consortium (S MART) collaboration, which
brought together all the tr ialists that have conducted the
relevant large tri als. The totality of the evidence prov ides
high levels of certainty of efficacy, w ith larger effect sizes
observed in many p opulatio ns. Relative effects on kidney
disease progression appeared to be larger among people
with higher leve ls of albuminuria who are at highest ab-
solute r isk of progression. The size of RR reductions
Mean
baseline eGFR,
ml/min per
1.73 m
2
Events/participants
SGLT2 inhibitor Placebo
Cardiovascular death or hospitalization for heart failure*
RR
(95% CI)
Diabetes
High atherosclerotic
cardiovascular risk trials
Stable heart failure trials
†
Chronic kidney disease trials
Subtotal: diabetes
No diabetes
Stable heart failure trials
†
Chronic kidney disease trials
Subtotal: no diabetes
Total: overall
80
61
45
67
64
40
56
65
1490/24,563
923/5046
643/10,474
3056/40,691
710/5316
50/2476
760/7792
3816/48,483
1232/18,005
1154/5037
847/10,457
3233/34,113
890/5322
53/2491
943/7813
4176/41,926
0.80 (0.74–0.86)
0.77 (0.71–0.84)
0.74 (0.66–0.82)
0.77 (0.73–0.81)
0.78 (0.70–0.86)
0.95 (0.65–1.40)
0.79 (0.72–0.87)
0.77 (0.74–0.81)
Heterogeneity by diabetes status: P=0.67
Events/participants
SGLT2 inhibitor Placebo
RR
(95% CI)
Cardiovascular death
1026/24,563
468/5046
363/10,474
1908/40,691
396/5316
26/2476
422/7792
2330/48,483
755/18,005
527/5037
434/10,457
1774/34,113
452/5322
25/2491
477/7813
2251/41,926
0.86 (0.78–0.95)
0.88 (0.78–0.99)
0.83 (0.72–0.95)
0.86 (0.80–0.92)
0.88 (0.77–1.00)
1.04 (0.59–1.83)
0.88 (0.78–1.01)
0.86 (0.81–0.92)
Heterogeneity by diabetes status: P=0.68
0.750.5 1.00 1.25 1.50
Noncardiovascular death
Diabetes
High atherosclerotic
cardiovascular risk trials
Stable heart failure trials
†
Chronic kidney disease trials
Subtotal: diabetes
No diabetes
Stable heart failure trials
†
Chronic kidney disease trials
Subtotal: no diabetes
Total: overall
80
61
45
67
64
40
56
65
572/24,557
317/5046
230/10,474
1133/40,685
263/5316
38/2476
301/7792
1434/48,477
461/18,003
316/5037
240/10,457
1035/34,111
251/5322
52/2491
303/7813
1338/41,924
0.88 (0.78–1.00)
1.00 (0.86–1.16)
0.94 (0.79–1.12)
0.93 (0.85–1.01)
1.05 (0.88–1.24)
0.74 (0.49–1.14)
1.00 (0.85–1.17)
0.94 (0.88–1.02)
Heterogeneity by diabetes status: P=0.43
All-cause death
1671/24 ,563
785/5046
599/10,474
3120/40,691
659/5316
64/2476
723/7792
3843/48,483
1299/18,005
843/5037
683/10,457
2901/34,113
703/5322
77/2491
780/7813
3681/41,926
0.87 (0.81–0.94)
0.93 (0.84–1.02)
0.87 (0.78–0.97)
0.88 (0.84–0.93)
0.94 (0.85–1.05)
0.84 (0.60–1.18)
0.93 (0.84–1.03)
0.89 (0.85–0.94)
Heterogeneity by diabetes status: P=0.36
SGLT2 inhibitor Favors placebo
0.750.5 1.00 1.25 1.50
SGLT2 inhibitor Favors placebo
Figure 23 | Effects of sodium-glucose cotransporter-2 (SGLT2) inhibition versus placebo on cardiovascular and mortality outcomes by
diabetes status and trial population. Collaborative meta-analysis of data from 13 large placebo control trials of SGLT2 inhibitors.
†
Data from
Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) are included in
totals but excluded from the stable heart failure trials group as the trial included patients with recent acute decompensated heart failure. CI,
confidence interval; eGFR, estimated glomerular filtration rate; RR, relative risk. Reproduced from Nuffield Department of Population Health
Renal Studies Group, SGLT Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-
transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–1801.
511
ª
2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
chapter 3 www.kidney-international.org
S216
Kidney International (2024) 105 (Suppl 4S), S117–S314

appears to be irrespective o f the level of GFR, w ith n o
evidence of a threshold level of eGFR below which ben-
efits start to attenuate.
For the 1A recommendation (3.7.1), also see the 2022
update to the KDIGO Clinical Practice Guideline in Diabetes
Management for det ails of the certainty of the evidence.
23
Our
ERT specifically also undertook a systematic review limited to
people with CKD and no diabetes and considered the
certainty of the effect in this subgroup to be moderate. The
ERT identified the collaborative meta-analysis,
511
which
included data from 2 RCTs evaluating an SGLT2i among
adults with CKD without diabetes.
403,513
Both RCTs were
considered to have a low risk of bias. The collaborative
meta-analysis harmonized the definition of CKD
progression among the trials. The certainty of the evidence
for CKD progression was graded as high (no concerns
regarding the risk of bias of the studies or the consistency,
directness, and precision of the results). The certainty of the
evidence for the kidney failure outcome in people with
CKD without diabetes was downgraded to moderate due to
imprecision (although clear benefits are demonstrated in
the CKD trials: Figure 24
518
). Neither RCT reported on the
critical outcome of hospitalizations for any cause in the
subgroup without diabete s.
Values and preferences. The Work Group judged that fully
informed people with CKD with an indication for an
SGLT2i would choose to receive SGLT2i for their proven
benefits on risk of CKD progression, AKI, and a range of
cardiovascular outcomes, their generally good safety profile,
and simplicity to implement (assuming local availability
and insurance coverage if required). SGLT2i also confer
health benefits that may motivate people with CKD due to
the reduced risk of hospitalization and serious hyper-
kalemia and uric acid levels, all of which are common
CKD complications.
Resource use and costs. Because of the high cost of KRT,
SGLT2i have been fo und to be cost-saving in the people with
CKD and diabetes recruited in the completed trials.
519
Generic SGLT2i are already available in some countries.
From a healthcare system perspective, reducing the cost
burden of hospitalizations and dialysis is highly desirable,
and QoL may be preserved longer from their avoidance.
Specifics as to whether people bear the costs of these
medications will be country-dependent.
Considerations for implementation. The Work Group
considered it safe to continue or even initiate an SGLT2i
when the eGFR falls below 20 ml/min per 1.73 m
2
and
continue their use until the time KRT is initiated (as was
the approach used in the large CKD population
RCT s
403,513,514
). We also considered that initiating SGLT2i
does not necessitate alteration of frequency of laboratory
monitoring. It is not routinely necessary to recheck blood
tests after initiating an SGLT2i in adults with CKD (see
Practice Point 3.7.3).
403
Reduced glomerular hyperfiltration resulting from
SGLT2i can result in a dip in eGFR which is reversible.
None of the large trials demonstrated an increased risk of
AKI in people treated with SGLT2i (Figure 22), and the
intervention does not induce hyperkalemia (an important
difference compared with inhibitors of the renin-
angiotensin-aldosterone pathway, which generally require
additional monitoring after initiation [Figure 21]).
Note that adults with polycystic kidney disease were
excluded from the large CKD trials testing SGLT2i.
Mean
baseline
eGFR (ml/min/
1.73 m
2
)
56
44
44
36
47
42
39
40
Diabetes
CREDENCE
SCORED
DAPA-CKD
EMPA-KIDNEY
Subtotal: DIABETES
No diabetes
DAPA-CKD
EMPA-KIDNEY
Subtotal: NO DIABETES
TOTAL: OVERALL 45
Relative risk
(95% CI)
SGLT2i Placebo Placebo
116/2202
NA/NA
77/1455
74/1525
267/5182
165/2199
NA/NA
109/1451
116/1515
390/5165
32/697
83/1779
115/2476
52/701
105/1790
157/2491
382/7658 547/7656
Events/participants Rate per 1000
patient-years
SGLT2i
20
26
24
29
37
39
0.68 (0.54, 0.86)
0.69 (0.51, 0.92)
0.59 (0.44, 0.79)
0.66 (0.56, 0.77)
24
25
39
31
0.56 (0.36, 0.87)
0.80 (0.60, 1.07)
0.72 (0.56, 0.91)
0.67 (0.59, 0.77)
Heterogeneity by diabetes status: P=0.54
Trend across
trials sorted
by eGFR
P=0.48
P=0.19
0.750.5 1.00 1.25 1.50
SGLT2i better Placebo better
Figure 24 | Effects of sodium-glucose cotransporter-2 (SGLT2) inhibition versus placebo on kidney failure (chronic kidney disease
[CKD] trials). Kidney failure defined as a composite of sustained estimated glomerular filtration rate (eGFR) <15 ml/min per 1.73 m
2
(or
eGFR <10 ml/min per 1.73 m
2
in The Study of Heart and Kidney Protection With Empagliflozin [EMPA-KIDNEY]), maintenance dialysis, or kidney
transplantation. Data for kidney failure not available for Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients with Type 2
Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED).
518
CI, confidence interval; CREDENCE, Canagliflozin and
Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes
in Chronic Kidney Disease; NA, not applicable.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S217
Rationale
Large trials individually and when combined in meta-analysis
demonstrate clear net benefits of SGLT2i, with net benefits
particularly large in people without diabetes due to almost no
risk of serious harm from ketoacidosis or lower-limb ampu-
tation.
Recommendation 3.7.3: We suggest treating adults
with eGFR 20 to 45 ml/min per 1.73 m
2
with urine
ACR <200 mg/g (<20 mg/mmol) with an SGLT2i
(2B).
This recommendation places high value on the potential for long-
term use of SGLT2i in people without diabetes who have a sub-
stantially decreased GFR to reduce the risk of kidney failure but
recognizes remaining uncertainty in this population due to the
short follow-up in the RCTs. It also places moderate value on the
benefits of SGLT2i on risk of AKI, cardiovascular death and
myocardial infarction, and risk of hospitalization from any cause.
SGLT2i also favorably reduce BP, uric acid levels, fluid overload,
and the risk of serious hyperkalemia. Note that a person with CKD
and heart failure has a clear indication for the use of SGLT2i to
reduce risk of cardiovascular de ath or hospitalization for heart
failure irrespective of level of albuminu ria (Figure 24).
Key information
Benefits and harms.
Several large placebo-controlled R CTs
have provided clear demonstrations of the efficacy of SGLT2i,
which substantially reduce the risk of kidney disease progression
and kidney failure (Figures 22 and 24)aswellasmoderately
reduce the risk of CVD events (Figure 23) in people with and
without CKD. Furthermore, a meta-analysis of the kidney
disease progression outcome subdivided by primary kidney
diagnosis demonstrated that there was no significant subgroup
interaction by primary kidney diagnosis, and SGLT2i reduced
the risk of AKI by 23% in people with or without diabetes
(Figure 22).
511
SGLT2i also reduce the risk of hospitalization
for any cause in people with CKD.
403,520
Some uncertainty
remains about the effects on kidney disease progression in
people without diabetes with urine ACR <200 mg/g (<20 mg/
mmol), which led to a different grading of the
recommendation for that population. EMPA-KIDNEY was the
key trial to assess effects in people with CKD at risk of
progression w ith urine ACR <200 mg/g (<20 mg/mmol) and
found evidence of significant interaction by ACR status for its
primary outcome (trend P ¼ 0.02). Relative effects appeared to
be larger in people with higher levels of albuminuria. The slow
rate of progression and small number of outcomes in the A1
subgroup limited the power for EMPA-KIDNEY to assess
effects on the primary outcome in this subgroup. There were,
however, important effects on the chronic (i.e., long-term)
slope in all albuminuria subgroups, and significant reductions
in progression using total slope analyses over the 2 years of
follow-up in the A2 and A3 groups were considered separately
(Figure 25
403
).
Certainty of evidence. The overall certainty of evidence for
the efficacy of SGLT2i to delay CKD progression in people with
CKD without diabetes is moderate (see Supplementary Table
S10
23,403,511,518,521–524
). The ERT identified an individual
participant data (IPD) meta-analysis,
511
which included data
from 2 RCTs evaluating an SGLT2 inhibitor among adults
with CKD but not diabetes.
403,513
Both RCTs were considered
to have a low risk of bias. The IPD meta-analysis harmonized
the definition of CKD progression among the trials. The
certainty of the evidence for CKD progression was graded as
high as there were no concerns regarding the risk of bias of
the studies or the consistency, directness, and precision of the
results. The certai nty of the evidence for kidney failure was
downgraded to moderate due to imprecision.
Values and preferences. The Work Group judged that fully
informed adults without diabetes and low levels of albuminuria
(urine ACR <200 mg/g [<20 mg/mmol]) who have established
CKD and an eGFR of 20–45 ml/min per 1.73 m
2
may be
particularly motivated to take SGLT2i for the benefits identified
on rate of decline in GFR as they already have substantially
reduced GFR. Adults with established CKD are highly likely to
want to start treatment early to maximize benefits. Extrapola-
tion of the findings from eGFR slope analyses (Figure 25) could
mean substantial delays in any future requirement for KRT.
People with CKD may also be motivated by the potential for
SGLT2i to reduce risk of AKI, hospitalization, serious
hyperkalemia, fluid overload, and uric acid levels, all of which
are common CKD complications.
Resource use and costs. Health economic analyses are
required in people with CK D without diabetes and low levels
of albuminuria to establish their level of cost-effectiveness.
From a healthcare system perspective, reducing the cost
burden of hospitalizations and dialysis is highly desirable, and
QoL may be preserved longer from their avoidance. Specifics
as to whether people bear the costs of these medications will
be country-dependent.
Considerations for implementation.. The considerations for
implementation in people with CKD and low levels of albu-
minuria are no different to people with albuminuria (see
above for details).
Rationale
Large trials considered individually and combined in meta-
analysis demonstrate clear net benefits of SGLT2i, but ev-
idence for benefits on CKD progression in people without
diabetes and with low levels of albuminuria is limited to
eGFR slope analyses in heart failure trials and one CKD
trial all w ith relatively short follow-up periods. However,
extrapolation of these eGFR slope results suggests that
important benefits would accrue for such people if treated
long term.
chapter 3 www.kidney-international.org
S218
Kidney International (2024) 105 (Suppl 4S), S117–S314

Special considerations
Pediatric considerations.
SGLT2i have not been tested in
clinical trials on children with kidney disease. Limited
observational data and phase II trial data exist for children
with and without kidney disease. Four studies (99 children
and young adults with diabetes and normal GFR) found that
pharmacokinetics and pharmacodynamics were likely to be
the same in children and adults.
525–528
Recent work modeled
pediatric dapagliflozin dosing fo r smaller children based on
known pharmacokinetics and pharmacodynamics.
483
Side
effects reported from the prior studies included an increase
in glycosuria and infrequent reporting of nausea, genital
infection, dehydration, and abdominal pain. In an RCT,
there were no episodes of diabetic ketoacidosis and similar
numbers of hypoglycemia between placebo and
dapagliflozin, mostly occurring in those on insulin.
529
There is limited research on kidney effects of SGLT2i in
children. One study of 8 children with CKD and proteinuria
found a reduction in 24-hour urine protein from a mean of 2.1
g/d to a mean of 1.5 g/d over 12 weeks.
530
Theoretically, the
glycosuric effect of SGLT2i may lead to a negative calorie
balance, interfering with optimal growth, especially in small
children with underlying growth retardation. Clinical trials in
the pediatric population are suggested, including in those
with specific etiologies and at different age groups (i.e.,
prepubescent, peripubescent, and postpubescent).
3.8 Mineralocorticoid receptor antagonists (MRA)
The Work Group highlights a key recommendation and practice
points from the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease.
23
–1 –0.5 0 0.5 1 1.5 2
Mean annual rate of change in estimated GFR
(ml/min per 1.73 m
2
per year)
Empagliozin Placebo Absolute dierence
(95% CI)
Placebo better Empagliozin better
Diabetes
Present
–2.01 (0.11)
–2.30 (0.10)
–2.91 (0.11)
–2.92 (0.10)
0.90 (0.59, 1.21)
0.62 (0.33, 0.91)
Absent
–2.64 (0.13)
–2.59 (0.11)
–4.04 (0.17)
0.51 (0.15, 0.87)
0.73 (0.42, 1.05)
1.21 (0.76, 1.67)
Estimated GFR (ml/min per 1.73 m
2
)
<30
–2.12 (0.13)
≥30 <45
–1.86 (0.11)
≥45
–2.83 (0.16)
Urinary albumin-to-creatinine ratio (mg/g)
<30
–0.72 (0.16)
–1.19 (0.13)
–3.22 (0.10)
–0.88 (0.16)
–1.64 (0.13)
–4.42 (0.10)
0.17 (–0.27, 0.60)
0.46 (0.09, 0.83)
1.19 (0.92, 1.47)
≥30 ≤300
>300
All participants –2.16 (0.08) –2.92 (0.08) 0.75 (0.54, 0.96)
Diabetes
Present
–1.05 (0.12)
–1.66 (0.11)
–2.73 (0.12)
–2.75 (0.11)
1.68 (1.36, 2.00)
1.09 (0.79, 1.39)
Absent
–2.85 (0.14)
–2.50 (0.12)
–3.60 (0.17)
1.01 (0.63, 1.39)
1.32 (0.99, 1.65)
2.01 (1.53, 2.49)
Estimated GFR (ml/min per 1.73 m
2
)
<30
–1.84 (0.14)
≥30<45
–1.18 (0.12)
≥45
–1.58 (0.17)
Urinary albumin-to-creatinine ratio (mg/g)
<30
–0.11 (0.17)
–0.49 (0.14)
–2.35 (0.11)
–0.89 (0.16)
–1.69 (0.14)
–4.11 (0.11)
0.78 (0.32, 1.23)
1.20 (0.81, 1.59)
1.76 (1.46, 2.05)
≥30 ≤300
>300
All participants –1.37 (0.08) –2.75 (0.08) 1.37 (1.16, 1.59)
Long-term slope
Subgroup
Total slope
Figure 25 | Effects of empagliflozin versus placebo on annual rate of change in estimated glomerular filtration rate (GFR) by key
subgroups in the Study of Heart and Kidney Protection With Empagliflozin (EMPA-KIDNEY). CI, confidence interval. Reproduced from The
New England Journal of Medicine, The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, et al. Empagliflozin in patients
with chronic kidney disease, volume 388, issue 2, Copyright ª 2023 Massachusetts Medical Society. Reprinted with permission from
Massachusetts Medical Society.
403
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S219

Recommendation 3.8.1: We suggest a nonsteroidal
mineralocorticoid receptor antagonist with proven
kidney or cardiovascular benefit for adults with T2D,
an eGFR >25 ml/min per 1.73 m
2
, normal serum
potassium concentration, and albuminuria (>30 mg/
g[>3 mg/mmol]) despite maximum tole rated dose
of RAS inhibitor (RASi) (2A).
Practice Point 3.8.1: Nonsteroidal MRA are most
appropriate for adults with T2D who are at high risk of
CKD progression and cardiovascular events, as
demonstrated by persistent albuminuria despite other
standard-of-care therapies.
Practice Point 3.8.2: A nonsteroidal MRA may be added
to a RASi and an SGLT2i for treatment of T2D and CKD
in adults.
Practice Point 3.8.3: To mitigate risk of hyper-
kalemia, select people with consistently normal serum
potassium concentrati on and monitor serum potassium
regularly after initiation of a nonsteroidal MRA
(Figure 26 ).
Practice Point 3.8.4: The choice of a nonsteroidal MRA
should prioritize ag ents with documented kidney or
cardiovascular benefits.
Practice Point 3.8.5: A steroidal MRA may be use d for
treatment of heart failure, hyperaldosteronism, or re-
fractory hypertension, but may cause hyperkalemia or a
reversible decline in glomerular filtration, particularly
among people with a low GFR.
MRAs reduce BP and albuminuria in people with CKD
531
and are part of recommended care for heart failure with
reduced ejection fraction.
532
The large Finerenone in
Reducing Kidney Failure and Disease Progression in Diabetic
Kidney Disease (FIDELIO-DKD)
533
and Finerenone in
Reducing Cardiovascular Mortality and Morbidity in Diabetic
Kidney Disease (FIGARO-DKD)
534
placebo-controlled trials
and their pooled analysis (FIDELITY)
535
demonstrated that
the nonsteroidal MRA (ns-MRA) finerenone reduced
cardiovascular risk in people with CKD and T2D (HR: 0.86;
95% CI: 0.78–0.95). The benefit was in large part due to a
22% reduction in the risk of hospitalization for heart failure
(HR: 0.78; 95% CI: 0.66–0.92), with no clear effect on stroke
(Figure 27).
535
These trials have some limitations on their
generalizability to all people with CKD at risk of progression,
given that study participants had an eGFR of $25 ml/min
per 1.73 m
2
and an ACR of $30 mg/g ($3mg/mmol),and
that people without diabetes were excluded.
Whether based on laboratory data or investigator reports,
finerenone approxi mately doubled the RR of hyperkalemia
compared with controls. However, risks were generall y low
and average increase in serum potassium was approximately
0.2–0.3 mEq from baseline values. The low absolute baseline
risk of hyperkalemia may be due to the selection of partic-
ipants w ith serum potassium <4.8 mmol/l and careful
algorithmic monitoring of potassium during follow-up.
Specific a nalyses of FIDELIO-DKD reported that 2.3% and
11.0% of par ticipants in the finerenone g roup withdrew or
interrupted treatment due to hyperkalemia (defined as
serum potassium >5.5 mmol/l), respectively, versus
0.9% and 5.2% for the placebo group.
535
Overall, in
FIDELITY, permanent treatment withdrawal for
hyperkalemia was 1.7% versus 0.6%. Hospitalization for
seri ous hyperkalemia was relativel y rare with a <1% excess
risk over 3 years.
536
Finerenone was also otherwise
generally well-tolerated with no excess r isk for serious AKI
identified in the 2 large trials. Further details are available
in the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease.
23
Trials asse ssing the effect of combining an SGLT2i and
finerenone compared with eithe r alone are ongoing (Clin-
icalTrials.gov Identifier: NCT05254002). Adequately pow-
ered, large-scale, clinical outcome, placebo-controlled trials
of steroidal and ns-MRA s have not been conducted in
people with causes of CKD not related to diabetes, but are
ongoing.
537
K
+
≤4.8 mmol/l K
+
4.9–5.5 mmol/l
K
+
>5.5 mmol/l
• Initiate nerenone
- 10 mg daily if eGFR 25–59 ml/min/1.73 m
2
- 20 mg daily if eGFR ≥60 ml/min/1.73 m
2
• Monitor K
+
at 1 month after initiation and then every 4
months
• Increase dose to 20 mg daily, if on 10 mg daily
• Restart 10 mg daily if previously held for hyperkalemia and
K
+
now ≤5.0 mmol/l
• Continue nerenone 10 mg or 20 mg
• Monitor K
+
every 4 months
• Hold nerenone
• Consider adjustments to diet or concomitant
medications to mitigate hyperkalemia
• Recheck K
+
• Consider reinitiation if/when K
+
≤5.0 mmol/l
Figure 26 | Serum potassium monitoring during treatment with a nonsteroidal mineralocorticoid receptor antagonist (MRA)
(finerenone). Adapted from the protocols of Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-
DKD) and Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD). The Work Group considers
these potassium thresholds to be conservative, and it may be considered appropriate to continue MRAs in people with potassium of 5.5–6.0 mmol/
l. This algorithm could be used for steroidal MRA. The US Food and Drug Administration (FDA) has approved initiation of K
þ
< 5.0 mmol/l. This
figure is guided by trial design and the FDA label and may be different in other countries. Serum creatinine/estimated glomerular filtration rate
(eGFR) should be monitored concurrently with serum potassium. Reproduced from Kidney Disease: Improving Global Outcomes Diabetes Work
Group. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102:S1–S127.
23
chapter 3 www.kidney-international.org
S220
Kidney International (2024) 105 (Suppl 4S), S117–S314

Special considerations
Pediatric considerations.
No relevant studies to inform this
guideline have been completed in children.
3.9 Glucagon-like peptide-1 receptor agonists
(GLP-1 RA)
The Work Group highlights a key recommendation and practice
point from the KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease.
23
Recommendation 3.9.1: In adults with T2D and CKD
who have not achieved individualized glycemic
targets despite use of metformin and SGLT2 inhib-
itor treatment, or who are unable to use those
medications, we recommend a long-acting GLP-1 RA
(1B).
Practice Point 3.9.1: The choice of GLP-1 RA should
prioritize agents wit h documented cardiovascular
benefits.
Results of the FLOW trial assessing effects of GLP-1 RA in
a dedicated CKD population are awaited. It is a definitive
assessment of semaglutide on kidney outcomes in 3534
people with CKD, albuminuria, and T2D.
537a
Nevertheless,
extrapolating current evidence from trials in pe ople with
T2D where kidney functi on was generally preserved
suggests GLP-1 RA safely improve glycemic control and
may reduce weight and risk of CVD in people with
CKD.
23,538
Meta-analysis of these large, placebo-controlled
cardiovascular outcome GLP-1 RA trials has shown reduced
MACE in people with prior CVD or at high risk.
538
The
size of RR reductions on cardiovascular risk appears similar
in people with or without decreased GFR.
538
Once
aggregated, GLP-1 RAs were shown to have modestly
reduced risk of hosp italization for heart failure (HR: 0.89;
95% CI: 0.82–0.92) and separately reduced risk of death
from any cause (HR: 0.88; 95% CI: 0.82–0.94).
538
The
KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease has recommended
that long-acting GLP-1 RAs are prioritized ahead of insulin
in people with T2D and CKD. GLP-1 RAs with proven
Outcome Finerenone (n = 6519) Placebo (n = 6507) Hazard ratio
(95% CI)
P value
a
Number of
patients with
event per 100
patient-years
Number of
patients
with event
(%)
Number of
patients with
event per 100
patient-years
Number of
patients
with event
(%)
Composite cardiovascular outcome
b
0.00180.86 (0.78–0.95)5.01939 (14.4)4.34825 (12.7)
322 (4.9) 0.0920.88 (0.76–1.02)1.84364 (5.6)1.61
173 (2.7) 0.360.91(0.74–1.12)0.97189 (2.9)0.88
1.01198 (3.0) 0.950.99 (0.82–1.21)1.02198 (3.0)
256 (3.9)
Death from cardiovascular causes
Nonfatal myocardial infarction
Nonfatal stroke
Hospitalization for heart failure
0.00300.78 (0.66–0.92)1.68
eGFR ≥57% composite kidney outcome
c
360 (5.5)
1.31
1.96
325 (5.0)
465 (7.1) 0.00020.77 (0.67–0.88)2.55
Sustained ≥40% decrease in eGFR from baseline
0.0390.84 (0.71–0.99)1.62297 (4.6)1.38254 (3.9)
0.040
e
0.80 (0.64–0.99)0.96188 (2.9)0.76151 (2.3)
0.026
e
0.81(0.67–0.98)1.29237 (3.6)1.06195 (3.0)
257 (3.9) 361 (5.5) <0.00010.70 (0.60–0.83)4.03
0.46
e
0.53 (0.10–2.91)0.024 (<0.1)0.012 (<0.1)
Kidney failure
End-stage kidney disease
d
Sustained decrease in eGFR to <15 ml/min/1.73 m
2
Sustained ≥57% decrease in eGFR from baseline
Renal death
eGFR ≥40% composite kidney outcome
f
854 (13.1) 0.00040.85 (0.77–0.93)
817 (12.5)
1.40
4.81
4.60
995 (15.3)
962 (14.8)
5.64
5.45 0.00020.84 (0.76–0.92)
Death from any cause 3.10614 (9.4)2.76552 (8.5)
0.89 (0.79–>1.00
g
)
0.051
e
Hospitalization for any cause 2836 (43.5) 19.04 2926 (45.0)
0.087
e
0.96 (0.91–1.01)19.91
Favors nerenone Favors placebo
0.5 1.0 2.0
Figure 27 | Effect of finerenone versus placebo on kidney and cardiovascular outcomes in pooled analyses from the Finerenone in
Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) and Finerenone in Reducing
Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease (FIGARO-DKD trials). CI, confidence interval; eGFR, estimated
glomerular filtration rate.
a
Statistical tests where P values are provided were exploratory in nature; therefore, no adjustment for multiplicity was
performed.
b
The composite of time to first onset of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for
heart failure.
c
The composite of time to first onset of kidney failure, sustained $57% decrease in estimated glomerular filtration rate from
baseline over $4 weeks, or renal death.
d
Initiation of chronic dialysis for $90 days or kidney transplantation.
e
Analyses for P values not
prespecified.
f
The composite of time to first onset of kidney failure, sustained $40% decrease in estimated glomerular filtration rate from
baseline over $4 weeks, or renal death.
g
P ¼ 1.001 to 3 decimal places. Reproduced from Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular
and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J.
2022;43:474–484.
535
ª The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology. This is an
Open Access article distributed under the terms of the Creative Commons Attribution-NonCommercial License (https://creativecommons.org/
licenses/by-nc/4.0/).
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S221

cardiovascular benefit that do not require dose adjustment in
CKD include liraglutide, semaglutide (injectable), and
dulaglutide.
23
3.10 Metabolic acidosis
As GFR decreases, the kidney’s ability to excrete hydrogen
ions and generate bicarbonate decreases, resulting in the
development of chronic metabolic acidosis. Metabolic
acidosis is observationally associated with increased risk of
protein catabolism, muscle wasting, inflammation, and other
complications such as impaired cardiac function and mor-
tality that are also associated with decreased eGFR.
539,540
The
causality of such associations remains to be demonstrated.
Definition and prevalence. Serum bicarbonate concentration
begins to fall progressively once eGFR falls below 60 ml/min
per 1.73 m
2
with reductions most evident in CKD stages G4–
G5 (Figure 28,
541
Tabl e 23). The adjusted adult prevalence of
serum bicarbonate < 22 mmol/l was 7.7% and 6.7% in those
with and without diabetes at stage G3, A1, respectively,
increasing to 38.3% and 35.9% by CKD stage G5, A3.
Practice Point 3.10.1: In people with CKD, consider use of
pharmacological treatment with or without dietary inter-
vention to prevent development of acidosis with potential
clinical implications (e.g., serum bicarbonate <18 mmol/l
in adults).
Practice Point 3.10.2: Monitor treatment for metabolic
acidosis to ensure it does not result in serum bicarbonate
concentrations exceeding the upper limit of normal and
does not adver sely affect BP control, serum potassium, or
fluid status.
The Work Group has not provided a graded recommen-
dation for the treatment of acidosis due to a lack of large-scale
RCTs supporting its use. In 2012, a 2B recommendation was
justified because alkali supplementation may be a promising
low-cost, high-benefit adjunct treatment for people with CKD
and may be accessible to all populations. This was based on an
RCT that had suggested potential kidney disease progression
and nutritional benefits with no important increase in BP or
heart failure complications.
1
However, since 2012, a number
of trials testing the hypothesis that sodium bicarbonate
therapy would slow kidney disease progression have been
reported, including several employing placebo control. A
2021 systematic review identified 15 trials with $3 months
of follow-up in peop le w ith CKD (eGFR <60 ml/min per
1.73 m
2
and/or proteinuria) comparing the effects of oral
sodium bicarbonate versus placebo or versus no study
medication on kidney outcomes. Of the 15 trials (2445
participants, median follow-up 12 months), 11 were
published since 2012. The totality of the evidence remains
limited by a low number of outcomes, and meta-analysis
restricted to the placebo-controlled trials does not confirm
any important modifying effect of oral sodium bicarbonate
versus placebo on risk of kidney failure (HR: 0.81; 95% CI:
0.54–1.22).
542
The largest placebo-controlled trial of oral
sodium bicarbonate was conducted by the Clinical and
cost-effectiveness of oral sodium bicarbonate therapy for
–5
–4
–3
–2
–1
1
0
15 30 45 60 75 90 105 12
0
eGFR, ml/min/1.73 m
2
Serum bicarbonate, mmol/l
A1
A2
A3
Figure 28 | Association between estimated glomerular filtration
rate (eGFR) with serum bicarbonate concentration in general
population and high-risk cohorts from the Chronic Kidney
Disease Prognosis Consortium, by level of albuminuria (A1–A3).
The y axis represents the meta-analyzed absolute difference from the
mean adjusted value at an eGFR of 80 ml/min per 1.73 m
2
and
albumin excretion <30 mg/g (<3 mg/mmol). Reproduced from
American Journal of Kidney Diseases, volume 73, issue 2, Inker LA,
Grams ME, Levey AS, et al. Relationship of estimated GFR and
albuminuria to concurrent laboratory abnormalities: an individual
participant data meta-analysis in a Global Consortium, pages 206–
217, Copyright ª 2018, with permission from the National Kidney
Foundation, Inc.
541
Table 23 | Variation of laboratory values in a large population database
a
by age group, sex, and eGFR; bicarbonate, mmol/l,
mean (SD), and n [ 3,990,898
Measure, mean (SD) Age (yr) Sex
GFR category (ml/min per 1.73 m
2
)
105D 90–104 75–89 60–74 45–59 30–44 15–29 0–14
Serum bicarbonate $65 Female 27.4 (4.1) 27.1 (2.9) 26.9 (2.9) 26.8 (2.9) 26.5 (3.1) 25.9 (3.5) 24.8 (4.0) 24.0 (4.8)
Male 27.1 (3.9) 26.6 (2.9) 26.7 (2.9) 26.5 (2.9) 26.1 (3.1) 25.3 (3.8) 24.1 (4.0) 24.2 (4.8)
<65 Female 25.2 (2.8) 26.1 (2.8) 26.3 (2.8) 26.4 (2.9) 26.2 (3.2) 25.1 (3.6) 23.6 (4.2) 24.0 (5.0)
Male 26.4 (2.8) 26.5 (3.0) 26.6 (2.7) 26.5 (2.9) 25.9 (3.2) 24.8 (4.4) 23.5 (4.1) 24.4 (4.7)
eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
a
Data from the Optum Labs Data Warehouse, a longitudinal, real-world data asset with deidentified administrative claims and electronic health record data. The database
contains longitudinal health information on enrollees and patients, representing the diversity of geographical regions across the United States.
chapter 3 www.kidney-international.org
S222
Kidney International (2024) 105 (Suppl 4S), S117–S314

older people with chronic kidney disease and low-grade
acidosis (BiCARB) Study Group.
543
It contributed 33 of 152
versus 33 of 148 kidney failure outcomes to the meta-
analysis in its bicarbonate versus placebo arms, respectively
(HR: 0.97; 95% CI: 0.64–1.49). Importantly, the BiCARB
trial, which studied people with CKD G3–G4 aged $60
years and sodium bicarbonate concentration <22 mmol/l,
also found no evidence of benefit on nonkidney outcomes
to support oral sodium bicarbonate supplementation (the
primary outcome was based on the Short Physical
Performance Battery at 12 months, and secondary outcomes
included generic and disease-specific QoL assessments,
anthropometry, kidney function, walk distance, BP, and
bone and vascular health markers). Allocation to oral
sodium bicarbonate was associated with higher costs and
lower European Quality of Life 5 Dimensions 3 Level
Version (EQ-5D-3L) assessed QoL over 1 year.
543
Licensed non-alkali oral interventions may be an alterna-
tive to oral sodium bicarbonate to treat metabolic acidosis but
have not been shown to have particular advantages.
544,545
Although placebo-controlled trials have found no good
evidence that cor recting sodium bicarbonate levels has
important effects on clinical outcomes, the Work Group
concluded that the intervention is clearly effective at
increasing serum bicarbonate concentration and is a
suitable treatment to avoid more severe acidosis with
potential for clinical implications. The Work Group suggests
that a serum bicarbonate of <18 mmol/l in adults is
desirable to avoid, but large RCTs are required to determine
a precise threshold whereby the treatment of low serum
bicarbonate levels leads to improvements in clinical
outcome. As correction of bicarbonate to the normal range
has not been demonstrated to reduce the risk of kidney
failure, lower thresholds to initiate therapy than 18 mmol/l
could be considered (see Research Recommendations).
Dietary approaches. Dietary modifications that limit the
consumption of acid-rich foods and/or increase the intake of
alkaline-rich foods reduce the net endogenous acid production
and can serve as an additional strategy to control metabolic
acidosis in people with CKD.
546,547
Such diets are generally low
in animal protein or have a higher consumption of plant-based
foods over animal-based foods (i.e., plant-dominant diets such
as Mediterranean or vegetarian diets). Four small RCTs of
alkaline-rich plant-based diets in adults with CKD
demonstrate a comparable benefittooralsodium
bicarbonate in controlling metabolic acidosis.
548–551
Special considerations
Pediatric considerations.
As in adults, children with CKD
often have metabolic acidosis. In the CKiD and the Cardio-
vascular Comorbidity in Children with Chronic Kidney Disease
Study (4C) studies, 38%–60% of children had a serum bicar-
bonate of <22 mmol/l, varying by CKD category. Low bicar-
bonate was associated with increased risk of disease
progression.
395,552
It should also be noted that for younger
children, the normal range for sodium bicarbonate is as low
as 17 mmol/l. In children, metabolic acidosis is also likely to
cause growth retardation. Data from the observational CKiD
study revealed that prepubertal children with acidosis who
were treated with alkali had improved growth.
553
In children
with normal GFR but renal tubular acidosis, prolonged
acidosis can also result in poor growth. The KDOQI
guideline on bone metabolism for children with CKD
recommends the prevention of acidosis in children to
optimize growth.
554
There have not been any trials examining
the effect of bicarbonate supplementatio n on CKD
progression or growth in children.
3.11 Hyperkalemia in CKD
Definition and prevalence. Potassium is key to cell mem-
brane electrophysiology, with abnormalities predisposing to
abnormal cardiac conduction and arrhythmias. The kidneys
play a key role in potassium homeostasis with decreased GFR
generally associated with increased potassium concentration
(Table 24; Figure 29
555
). The definition of hyperkalemia is
based on the dist ribution of potassium values in the general
population. Hyperkalemia is uncommon when the eGFR is
>60 ml/min per 1.73 m
2
and increases with lower GFR.
Adults with CKD G3, A1 in the general and high-r isk pop-
ulation cohorts, contributing to the CKD-PC, had an adjusted
prevalence of hyperkalemia (defined as a serum potassium
>5.0 mmol/l) of 8.8% and 4.5% in those with and w ithout
diabetes, respectively, increasing to 34.4% and 23.7% by CKD
G5, A3 (Figure 30).
541
Note that there is variability in the
prevalence of hyperkalemia, and it is not inevitable at lower
Table 24 | Variation of laboratory values in a large population database
a
by age group, sex, and eGFR; potassium, mmol/l, mean
(SD), and n [ 4,278,600
Measure, mean (SD) Age (yr) Sex
GFR category (ml/min per 1.73 m
2
)
105D 90–104 75–89 60–74 45–59 30–44 15–29 0–14
Serum potassium $65 Female 4.1 (0.5) 4.2 (1.3) 4.2 (0.5) 4.3 (0.5) 4.3 (1.3) 4.4 (0.5) 4.5 (1.0) 4.5 (2.0)
Male 4.2 (0.5) 4.3 (0.6) 4.3 (1.1) 4.4 (0.6) 4.4 (0.7) 4.5 (1.1) 4.6 (0.6) 4.6 (1.6)
<65 Female 4.1 (0.7) 4.2 (1.3) 4.3 (17.0) 4.2 (1.0) 4.3 (0.5) 4.3 (0.6) 4.4 (0.6) 4.5 (1.1)
Male 4.2 (0.4) 4.3 (0.5) 4.3 (0.6) 4.3 (0.4) 4.4 (0.5) 4.5 (0.6) 4.5 (0.7) 4.6 (0.7)
eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
a
Data from the Optum Labs Data Warehouse, a longitudinal, real-world data asset with deidentified administrative claims and electronic health record data. The database
contains longitudinal health information on enrollees and patients, representing the diversity of geographical regions across the United States.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S223

levels of GFR, thus understanding potassium physiology and its
impacting factors are important in effective patient care.
Hyperkalemia in people with preserved GFR is less prev-
alent. An acute episode of hyperkalemia is a potassium result
above the upper limit of normal that is not known to be
chronic. At the current time, there is no consensus on the
magnitude, duration, and frequency of elevated potassium
values that define chronicity.
556
In addition to decreased
eGFR, other risk factors for hyperka lemia included higher
ACR and prior diabetes, hyperglycemia, constipation,
RASi,
557
and MRA.
536
Note that SGLT2i do not appear to
increase serum potassium values.
403,517
Studies have demonstrated a continuous U-shaped rela-
tionship between serum potassium and all-cause mortality
in a range of different populations (Figure 31).
555,558
It has
also been associated with worse kidney prognosis.
559
Observationally, the risk of death from the same degree of
hyperkalemia is lower in more advanced CKD stages.
560–564
This may suggest that there are adaptive mechanisms that
render better tolerance to elevated levels of potassium in
circulation.
561,565–568
3.11.1 Awareness of factors impacting on potassium
measurement
There are several factors and mechanisms that may impact on
potassium measurements, including the actions of medications
that can increase the risk of developing hyperkalemia. These are
summarized in Tables 25
556,569–575
and 26.
23,576
Practice Point 3.11.1.1: Be aware of the variability of po-
tassium laboratory measurements as well as factors and
mechanisms that may influence potassium measurement
including diurnal and seasonal variation, plasma versus
serum samples, and the actions of medications.
The Work Group would like to highlight Figure 26 for the
monitoring of serum potassium during treatment w ith a
nonsteroidal MRA (finerenone) from the KDIGO 2022
Clinical Practice Guideline for Diabetes Management in
Chronic Kidney Disease.
23
Hyperkalemia has been associated with therapeutic actions
of either reducing or stopping RASi.
577–580
Steps can be taken
to mitigate the risk of hype rkalemia and improve potassium
control that could increase the use of RASi in people with an
evidenced indication. For details on how to manage hyper-
kalemia associated with the use of RASi and associated
>90
75–89
60–74
45–59
30–44
15–29
1.5% (0.4, 4.6)
1.7% (0.5, 5.1)
2.3% (0.7, 7.0)
4.5% (1.4, 12.8)
9.5% (3.0, 24.8)
16.1% (5.3, 37.5)
1.1% (0.3, 3.2)
1.6% (0.5, 4.8)
2.0% (0.6, 6.0)
3.5% (1.1, 10.3)
10.5% (3.3, 26.9)
19.0% (6.4, 42.5)
1.4% (0.4, 4.4)
1.5% (0.5, 4.7)
2.3% (0.7, 7.0)
5.2% (1.6, 14.6)
11.3% (3.6, 28.5)
23.7% (8.3, 49.4)
No diabetes
Hyperkalemia
eGFR A1 A2 A3
>90
75–89
60–74
45–59
30–44
15–29
1.8% (0.5, 5.5)
2.8% (0.8, 8.3)
3.9% (1.2, 11.2)
8.8% (2.7, 23.3)
12.8% (4.1, 31.5)
24.7% (8.8, 50.8)
3.6% (1.1, 10.5)
4.0% (1.2, 11.6)
5.0% (1.5, 14.2)
9.9% (3.1, 25.5)
18.7% (6.3, 41.9)
31.5% (11.9, 59.1)
1.0% (0.3, 3.0)
4.6% (1.4, 13.0)
6.6% (1.8, 17.4)
11.4% (3.6, 28.8)
87.5% (67.1, 95.6)
34.4% (13.3, 62.2)
Diabetes
Hyperkalemia
eGFR A1 A2 A3
Figure 30 | Meta-analyzed adjusted prevalence of hyperkalemia (25th and 75th percentile cohort) in general population and high-risk
cohorts from the Chronic Kidney Disease Prognosis Consortium, by diabetes status. Hyperkalemia is defined as potassium >5 mmol/l. The
adjusted prevalence of hyperkalemia at each estimated glomerular filtration rate (eGFR) and albuminuria stage was computed as follows: first,
the random-effects weighted adjusted mean odds at the reference point (eGFR 50 ml/min per 1.73 m
2
) was converted into a prevalence
estimate. To the reference estimate, the meta-analyzed odds ratios for hyperkalemia were applied to obtain prevalence estimates at eGFR 95,
80, 65, 35, and 20 ml/min per 1.73 m
2
for each stage of albuminuria. The prevalence estimates were adjusted to 60 years old, half male, non-
Black, 20% history of CVD, 40% ever smoker, and body mass index 30 kg/m
2
. The 25th and 75th percentiles for predicted prevalence were the
estimates from individual cohorts in the corresponding percentiles of the random-effects weighted distribution of adjusted odds. A1,
albuminuria <30 mg/g (<3 mg/mmol); A2, albuminuria 30–300 mg/g (3–30 mg/mmol); A3, >300 mg/g (>30 mg/mmol). Reproduced from
American Journal of Kidney Diseases, volume 73, issue 2, Inker LA, Grams ME, Levey AS, et al. Relationship of estimated GFR and albuminuria to
concurrent laboratory abnormalities: an individual participant data meta-analysis in a Global Consortium, pages 206–217, Copyright ª 2018,
with permission from the National Kidney Foundation, Inc.
541
0
0.2
0.4
0.6
0.8
1
Density
2.5 3 3.5 4 4.5 5 5.5 6 6.5
Potassium, mmol/
eGFR 60+
eGFR 30–59
eGFR <30
Figure 29 | Distribution of blood potassium in general population
and high-risk cohorts from the Chronic Kidney Disease Prognosis
Consortium, by estimated glomerular filtration rate (eGFR).
Density refers to the proportion of the population experiencing
serum potassium level (e.g., 0.08 of the population with a GFR >60
have a potassium of 3.8; conversely, 0.2 of the population with a
GFR <30 have a potassium of 5.5). Reproduced from Kovesdy CP,
Matsushita K, Sang Y, et al. Serum potassium and adverse outcomes
across the range of kidney function: a CKD Prognosis Consortium
meta-analysis. European Heart Journal 2018;39:1535–1542 by
permission of Oxford University Press on behalf of the European
Society of Cardiology.
555
All rights reserved. ª The Author(s) 2018.
Inclusion under a Creative Commons license is prohibited. https://doi.
org/10.1093/eurheartj/ehy100
chapter 3 www.kidney-international.org
S224
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table 25 | Factors and mechanisms that impact on potassium measurements
556,569-575
Factor/mechanism Possible cause/clinical implication
Pseudohyperkalemia—in vivo serum potassium is normal and
commonly GFR preserved, but during the process of
drawing blood or clotting, there has been a release of
intracellular potassium
Tight tourniquet
Hand/arm exercising or clenching at the time of blood draw
Hemolysis due to vigorous shaking of blood vial/inappropriate blood draw
equipment/inappropriate storage of samples
If suspected, blood should be retaken and analyzed in the appropriate
manner and time frame
556,569
Presence of thrombocytosis/leukocytosis
If suspected, take plasma potassium as serum potassium may be falsely
increased
570
Hyperkalemia due to disruption in the mechanism of shifting
potassium out of cells
Increase in plasma osmolarity (e.g., dehydration and hyperglycemia)
Massive tissue breakdown (e.g., rhabdomyolysis and tumor lysis syndrome)
b
-adrenergic blockade, especially during and immediately after exercise
569
Insulin deficiency
Aldosterone blockade
Nonorganic acidosis
Hyperkalemia due to disruption in the mechanism of moving
potassium into cells
Disruption in the release of insulin in response to raised serum potassium (e.g., in
uncontrolled diabetes)
Disruption to the release of aldosterone in response to a raised serum potassium
569
Hyperkalemia due to the decreased ability to excrete
potassium
Advancing CKD resulting in inability to excrete excessive potassium
Constipation: in advancing CKD, the gut assumes a much more important role in
maintaining potassium balance by increasing the excretion of potassium
571,572
Medications: blocking the RAAS pathway and other medication resulting in the
inability to excrete excessive potassium (Table 26)
569,573
Diurnal variation in potassium excretion with most excretion
in humans occurring close to noon
Circadian excretion of kidney electrolytes have been well documented.
574
Clinical
relevance is yet to be understood
Note the 0.24–0.73 mmol/l variation in Kþ values within individuals over a
24-hour period
Plasma vs. serum potassium values
Potassium values differ between serum and plasma values with serum values being
typically higher. Healthcare providers need to be aware of the right reference
values for the sample
570
Postprandial hyperkalemia As kidney function declines in CKD, there is a corresponding decline in the ability of
the kidneys to increase kaliuresis postprandially, eventually becoming
insufficient to maintain external potassium balance
575
CKD, chronic kidney disease; GFR, glomerular filtration rate; Kþ, potassium; RAAS, renin-angiotensin-aldosterone system.
8.07.57.06.56.05.55.04.54.03.53.02.5
Baseline serum potassium level, mEq/l
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
Predicted probability of mortality
HF, CKD, and DM
CKD
HF
DM
Control group
Figure 31 | Serum potassium concentration and confounder-adjusted risk of death by the presence or absence of diabetes, heart
failure (HF), or chronic kidney disease (CKD). Reproduced from Collins AJ, Pitt B, Reaven N, et al. Association of serum potassium with all-
cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221.
558
ª 2017
The Author(s) Published by S. Karger AG, Basel. This article is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives
4.0 International License (CC BY-NC-ND) (http://www.karger.com/Services/OpenAccessLicense).
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S225

monitoring, please refer to Figure 21. See Section 4.3 for more
information on continuing RASi after hyperkalemia events.
3.11.2 Potassium exchange agents
Practice Point 3.11.2.1: Be aware of local availability or
formulary restrictions with regard to the pharmacologic
management of nonemergent hyperkalemia.
The pharmacologic management of nonemergent hyper-
kalemia has a number of clinical tools with the increased
number of licensed potassium exchange agents. These med-
ications have differing mechanisms of action, onsets of clin-
ical effects, and potential medication and disease-state
interactions (Table 27
581,582
). Although the classic potassium
exchange agents have had tolerability issues, the newer ones
appear to have fewer such issues and appear relatively safe
when used long term.
575,583,584
Use of these newer exchange
agents may help facilitate essential use of RASi/MRA.
However, it is important that the healthcare provider be
aware of clinical nuances and local availability or formulary
restrictions in determining therapy selection.
583
A
comparison of available potassium exchange agents can be
found in Table 27.
3.11.3 Timing to recheck potassium after identifying moder-
ate and severe hyperkalemia in adults
“Think Kidneys” and the UK Kidney Association have pro-
vided a practical guide, which we have adapted (Tabl e 2 8)for
repeat testing after a hyperkalemic episode.
585
The timing of
repeat testing is guided by the level of hyperkalemia and the
clinical context.
586
3.11.4 Managing hyperkalemia
In people w ith CKD and the management of nonemergent
hyperkalemia, a systematic approach of treating correctable
factors (e.g., correction of severe metabolic acidosis) and
understanding the role of diet and medications may provide
a prag matic framework. Figure 32 shows a stepwi se
practical approach to the management of hyperkalemia in
CKD.
3.11.5 Dietary considerations
In early stages of CKD, high intake of foods naturally rich in
potassium appears to be protective against disease progres-
sion, and dietary restriction of foods naturally containing
potassium, such as fruits and vegetables, may be harmful to
cardiac health; therefore, such restriction is not endorsed.
587
Practice Point 3.11.5.1: Implement an individualized
approach in people with CKD G3 – G5 and emergent
hyperkalemia that includes dietary and pharmacologic in-
terventions and takes into consi deration associated
comorbidities and quality of life (QoL). Assessment and
education through a renal dietitian or an accredited
nutrition provider are advised.
Practice Point 3.11.5.2: Provide advice to limit the intake of
foods rich in bioa vailable potassium (e.g., processed foods)
for people with CKD G3–G5 who have a history of
hyperkalemia or as a prevention strategy during disease
periods in which hyperkalemia risk may be a concern.
Diet may increase serum potassium postprandially,
575,588
but other conditions such as the use of potassium-sparing
Table 26 | Medications associated with increased risk of hyperkalemia
Class Mechanism Example
ACEi Inhibit conversion of angiotensin I to angiotensin II Captopril, lisinopril, perindopril, etc.
ARB Inhibit activation of angiotensin I receptor by angiotensin II Losartan, irbesartan, candesartan, etc.
Aldosterone antagonist Block aldosterone receptor activation Spironolactone, eplerenone, and finerenone
b
-Adrenergic receptor
blocker
Inhibit renin release Propranolol, metoprolol, and atenolol
Digitalis glycoside Inhibit Na
þ
-K
þ
-ATPase, necessary for collecting duct K
þ
secretion Digoxin
Heparin Reduced production of aldosterone Heparin sodium
Potassium-sparing
diuretic
Block collecting duct apical Na
þ
channel, decreasing gradient for K
þ
secretion Amiloride and triamterene
NSAIDs Inhibit synthesis of prostaglandin E and prostacyclin, inhibiting renin release Ibuprofen, naproxen, diclofenac, etc.
CNI Inhibit Na
þ
-K
þ
-ATPase, necessary for collecting duct K
þ
secretion Cyclosporine and tacrolimus
ns-MRA Block MR-mediated Na
þ
reabsorption Finerenone
Other Block collecting duct apical Na
þ
channel, decreasing gradient for K
þ
secretion Trimethoprim and pentamidine
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; ATP, adenosine triphosphate; CNI, calcineurin inhibitor; K
þ
, potassium; Na
þ
, sodium;
NSAID, nonsteroidal anti-inflammatory drug; ns-MRA, nonsteroidal mineralocorticoid receptor antagonist.
Data from Weiner et al.
576
and Kidney Disease: Improving Global Outcomes Diabetes Work Group.
23
chapter 3 www.kidney-international.org
S226
Kidney International (2024) 105 (Suppl 4S), S117–S314

medications, metabolic acidosis, hyperosmosis due to
hyperglycemia, hypernatremia or uremia, and constipation
are more likely to explain plasma potassium abnormalities
than diet.
545,556,572,589
Although short-term dietary
restriction of the foods highest in potassium is a valid
strategy to treat acute hyperkalemia, restriction of foods
highest in bioavailable potassium may be a supportive
prevention strategy for people w ith a history of
hyperkalemia or during periods in which hyperkalemia risk
is a concern.
590
Increased efforts toward education on
potassium content in foods can serve to improve diet
quality and diversity for many people with CKD where this
Table 27 | A comparison of potassium exchange agents
(Polystyrene sulfonates) sodium or calcium Patiromer Sodium zirconium cyclosilicate (SZC)
Mechanism of
action
Sodium-potassium exchange resin (SPS) or
calcium-potassium exchange resin (CPS)
Calcium-potassium exchange polymer Crystalline compound that traps K
þ
in
exchange for hydrogen and sodium
cations
Counterion
content
SPS: Suspension contains 65 mmol/60 ml (15 g)
of sodium and powder approximately 4.1
mmol/g of sodium.
CPS: 1.6–2.4 mmol/g of calcium.
1600 mg of calcium per 8.4 g of
patiromer
Approximately 400 mg of sodium per
5 g of SZC
Cations bound Potassium, magnesium, and calcium Potassium, magnesium, and phosphate
(bound by calcium release)
582
Potassium
Formulation
of route of
administration
Powder for reconstitution (oral), suspension
(oral), and enema (rectal)
Powder for reconstitution (oral) Powder for reconstitution (oral
suspension)
Dosage and
titration
Oral: 15–60 g/d (up to 4 times per day)
Rectal: 30 g/d (for SPS up to a maximum
of 50 g/d)
Initial: 8.4 g orally once per day
(maximum 25.2 g orally once per
day); dose can be increased by 8.4 g
increments at 1-week intervals
Initial: 10 g orally 3 times per day for
up to 48 hours
Maintenance
dosing
15–60 g/d orally per day depending on
potassium level and level of tolerability
8.4–25.2 g orally once per day 5 g every second day to 10 g once
per day
Onset of effect Variable, hours to days 4–7 hours Starts to reduce potassium within
1 hour with normokalemia typically
at 24–48 hours
Duration
of effect
Variable, 6–24 hours 24 hours Not studied; not systematically
absorbed and excreted fecally
Administration
pearls
Separate from oral medications by at least
3 hours before or 3 hours after
administration; if gastroparesis, separate
other medications by 6 hours
Separate from oral medications by at
least 3 hours before or 3 hours after
administration except for those
drugs to not have a clinically
important interaction
No dose adjustments or separation of
time of dosing is required for any
medication that does not have pH-
dependent bioavailability. However,
SZC should be administered at least
2 hours before or 2 hours after oral
medicinal products with clinically
meaningful gastric pH-dependent
bioavailability
Adverse effects GI events (nausea, vomiting, diarrhea,
constipation), electrolyte disturbances
(hypokalemia, hypocalcemia, and
hypomagnesemia), edema, and potentially
serious GI adverse events (intestinal
necrosis, bleeding, ischemic colitis, and
perforation)
GI events (nausea, diarrhea, and
flatulence), electrolyte disturbances
(hypokalemia, hypercalcemia, and
hypomagnesemia)
Insufficient postmarketing surveillance
at present to evaluate long-term/
rare events
Hypokalemia and edema events are the
most common. Milder reports of GI
events (nausea, diarrhea, and
constipation)
Insufficient postmarketing surveillance
at present to evaluate long-term/
rare events
GI, gastrointestinal.
Modified from Bridgeman MB, Shah M, Foote E. Potassium-lowering agents for the treatment of nonemergent hyperkalemia: pharmacology, dosing and comparative efficacy.
Nephrology Dialysis Transplantation, Volume 34, Supplement 3, pages iii45–iii50.
581
ª The Author(s) 2019. Published by Oxford University Press on behalf of ERA-EDTA. All
rights reserved.
Table 28 | Suggested action in the event of moderate and
severe hyperkalemia
Severity of
hyperkalemia Clinically unwell or AKI Unexpected result
Moderate
K
þ
6.0–6.4 mmol/l
Assess and treat
in hospital
Repeat within
24 hours
Severe
K
þ
$6.5 mmol/l
Take immediate action to assess and treat.
Assessment will include blood testing and
electrocardiogram monitoring
AKI, acute kidney injury; K
þ
, potassium.
Data from Think Kidneys, the Renal Association and the British Society for Heart
Failure.
585
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S227

restriction may not be needed.
546,556,591
Although guidelines
and available information to people with CKD have heavily
emphasized plant-based foods as potential causes of
hyperkalemia in CKD,
592
other healthy nutrients in plant-
based foods affect potassium absorption and
distribution
588,593,594
; therefore, the net bioavailable
potassium from plant-based foods may be lower than
appreciated.
595
Highly processed foods (rich in potassium
additives), meats, dairy products, juices, and salt substitutes
made with potassium chloride are actually higher in
absorbable potassium than many plant-based fresh foods
(Figure 33
592
).
596–598
Teaching materials for people with CKD should place a
greater focus on highly processed versus unprocessed food
restriction for hyperkalemia management.
592
An example of
a patient resource for potassium management can be
found at: http://www.bcrenal.ca/resourcegallery/Documents/
Potassium_Management_in_Kidney_Disease.pdf.
Cooking methods such as soaking foods for 5–10 minutes
in previously boiled water can effectively reduce the potas-
sium by half for some foods.
599
Thus, educating people
with CKD and healthcare providers, using clear messaging,
on dietary approaches to potassium management is
needed (https://www.theisn.org/initiatives/toolkits/raasi-toolkit/
#1684867542809-330edb7 9-52b4), as well as a policy to
improv e food labeling by detailing the added potassium used
in processing.
Special considerations
International considerations.
For people with CKD and
severe recurrent hyperkalemia (potassium >6 mmol/l), the
balance to be considered is between the additional cost of
the number needed to treat with potassium exchange agents
to prevent additional costs of hyperkalemia over and above
CKD management costs. If the price for potassium-lowering
therapy is lower than the reduction of inpatient and
outpatient costs due to prevented hyperkalemia, the cost-
benefit ratio will be favorable because in addition to the
health benefits, there is a net saving of healthcare costs
resulting from potassium-lowering treatment. The key is to
implement a successful affordable strategy for hyperkalemia
management that allows the maintenance of other therapies
3rd line:
Last resort
1st line:
Address correctable factors
2nd line:
Medications
• Review non-RASi medications (e.g. NSAIDs, trimethoprim)
• Assess dietary potassium intake (dietary referral) and consider
appropriate moderation of dietary potassium intake
Consider:
• Appropriate use of diuretics
• Optimize serum bicarbonate levels
• Licensed potassium exchange agents
• Reduce dose or discontinue RASi/MRA
(Discontinuation is associated with increased cardiovascular events.
Review and restart RASi or MRA at a later date if patient condition allows.)
Figure 32 | Actions to manage hyperkalemia (potassium >5.5 mmol/l) in chronic kidney disease. MRA, mineralocorticoid receptor
antagonists; NSAID, nonsteroidal anti-inflammatory drug; RASi, renin-angiotensin system inhibitors.
Plant-based foods
Absorption rate
50%–60%
Plant-based foods may have
and carbohydrate content encourages K
+
shifts into intracellular space, minimizing
impacts on serum K
+
Animal-based foods
Absorption rate
70%–90%
Animal-based protein has higher
in higher amounts of K
+
remaining
in serum
Processed foods
Absorption rate
90%
Potassium salts (often found in
processed foods) absorption rate
has been reported to be 90%
Figure 33 | Potassium absorption rates of plant-based, animal-based, and processed foods. Data from Picard K, Griffiths M, Mager DR,
Richard C. Handouts for low-potassium diets disproportionately restrict fruits and vegetables. J Ren Nutr. 2021;31:210–214.
592
chapter 3 www.kidney-international.org
S228
Kidney International (2024) 105 (Suppl 4S), S117–S314

directed at reducing both progression of CKD and reduction
in MACE.
Pediatric considerations. As described for adults with CKD,
abnormal serum potassium levels are also commonly seen in
children with advanced stages of CKD, as well as those with
glomerular disorders, metabolic acidosis, and those on
RASi.
600
In addition, a small group of children with CKD can
have persistent hypokalemia, usually as a result of inherited or
acquired renal tubular disorders.
In children with CKD, discontinuation of RASi was asso-
ciated with an acceleration of kidney function decline
compared with a matched control cohort of children in
whom RASi were continued.
395
In children with CKD, the dietary management of potas-
sium can pose unique challenges as the provision of adequate
energy, protein, and micronutrients for growth cannot be
compromised, and specialized low potassium nutritional
formulas may not be w idely available or palatable.
601
An example of a patient resource (for children and their
caregivers) for potassium management can be found at:
Nutrition taskforce—European Society for Paediatric
Nephrology (https://www.espn-online.org/ ).
3.12 Anemia
The KDIGO 2012 Clinical Practice Guideline for Anemia in
Chronic Kidney Disease will be updated in 2024.
436
Mean hemoglobin is, on average, lower in both men and
women with an eGFR <60 ml/min per 1.73 m
2
compared
with health adults and progressively falls with decreasing GFR
(Table 29; Figure 34
541
). For example, adults with CKD G3,
A1 in the general and high-risk population cohorts
contributing to the CKD-PC had an adjusted prevalence of
anemia (hemoglobin <12 g/dl in men; <11 g/dl in women)
of 14.9% and 11.5% in those with and without diabetes,
respectively, and this prevalence increased to 60.7% and
57.4% by CKD G5, A3. Note that a drop in Hb is expected
in pregnancy (physiologic anemia) and may not warrant
treatment (although the cutoff at which treatment is
desirable is unclear and requires clinical judgment). Please
refer to the KDIGO Clinical Practice Guideline for Anemia
in Chronic Kidney Disease publications for specific
recommendations, selection, and dosing of specific
therapeutic agents, as well as research recommendations.
3.13 CKD-Mineral Bone Disorder (CKD-MBD)
The Work Group highlights the KDIG O 2017 Clinical Practice
Guideline Update for the Diagnosis, Evaluation, Prevention,
and Treatment of Chronic Kidney Disease–Mineral and Bone
Disorder (CKD-MBD).
20
Please refer to this publication for
specific recommendations, selection, dosing of specific
therapeutic agents, and research recommendations.
Changes in bone mineral metabolism and alterations in
calcium and phosphate homeostasis occur early in the course
of CKD and progress as eGFR declines (Figure 35
541
). These
are detectable as abnormalities of serum calcium, phosphate,
vitamin D metabolites, and circulating hormones (i.e.,
parathyroid hormone [PTH] and fibroblast growth factor-
23). These changes are grouped under the umbrella term
CKD-MBD, which also includes renal osteodystrophy and
Table 29 | Variation of laboratory values in a large population database
a
by age group, sex, and eGFR; hemoglobin, g/dl, mean
(SD), and n [ 3,561,622
Measure, mean (SD) Age (yr) Sex
GFR category (ml/min per 1.73 m
2
)
105D 90–104 75–89 60–74 45–59 30–44 15–29 0–14
Hemoglobin $65 Female 12.2 (2.0) 13.2 (4.6) 13.2 (1.7) 13.2 (1.5) 12.8 (1.6) 12.1 (1.7) 11.2 (1.8) 10.3 (1.7)
Male 12.9 (2.4) 14.2 (1.8) 14.2 (1.7) 14.1 (1.8) 13.5 (1.9) 12.7 (2.0) 11.5 (2.0) 10.5 (2.0)
<65 Female 13.0 (1.4) 13.3 (1.3) 13.4 (2.0) 13.4 (1.4) 13.0 (1.6) 12.1 (1.8) 11.0 (1.9) 10.6 (2.5)
Male 14.9 (1.5) 15.0 (3.1) 15.0 (1.4) 14.9 (1.6) 14.1 (2.0) 12.9 (2.2) 11.7 (2.2) 10.9 (2.0)
eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
a
Data from the Optum Labs Data Warehouse, a longitudinal, real-world data asset with deidentified administrative claims and electronic health record data. The database
contains longitudinal health information on enrollees and patients, representing the diversity of geographical regions across the United States.
–2.5
–2
–1.5
–1
–0.5
0
0.5
1
1.5
Hemoglobin, g/d
15 30 45 60 75 90 105 12
0
eGFR, ml/min/1.73 m
2
Female
Male
Figure 34 | Association between estimated glomerular filtration
rate (eGFR) and hemoglobin concentration from general
population and high-risk cohorts from the Chronic Kidney Disease
Prognosis Consortium, by diabetes status. The y axis represents
the meta-analyzed absolute difference from the mean adjusted value
at an eGFR of 80 ml/min per 1.73 m
2
and albumin excretion <30 mg/g
(<3 mg/mmol). Reproduced from American Journal of Kidney Diseases,
volume 73, issue 2, Inker LA, Grams ME, Levey AS, et al. Relationship of
estimated GFR and albuminuria to concurrent laboratory abnormalities:
an individual participant data meta-analysis in a Global Consortium,
pages 206–217, Copyright ª 2018, with permission from the National
Kidney Foundation, Inc.
541
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S229

extraskeletal (i.e., vascular) calcification related to these
abnormalities of metabolism. It has been recommended
that in people with CKD G3a–G5, treatments of CKD-MBD
should be based on serial assessments of phosphate,
calcium, and PTH levels considered together.
20
Higher serum phosphate concentrations ar e associated with
mortality,
602
and experimental data suggest that serum phosphate
conce ntration is directly related to bone disease, vascular
calcification,
603,604
and CVD . Low-phosp horus diets and
binders are used to help lower serum phosphate to reduce the
long-term complications of CKD-MBD, although more
resear ch is needed to fully understand the disease-modifying
impact of these interventions.
605
Similarly, despite evidence
suggesting no benefitonclinicaloutcomes,
606
vitamin D
replac ement and calcimimetics to control PTH levels and to
maintain calcium within the normal range are also common
strategies. For recommenda tions regarding selection and dosing
with specific therapeutic agents and research, please see the
KDIGO 2017 Clinical Practice Guideline U pdate for the
Diagnosis, Ev aluation , Prevention, and Treatment of Chronic
Kidney Disease–Mineral and Bone Disord er (CKD-MBD).
20
3.14 Hyperuricemia
Definition and prevalence. Uric acid is the end product of the
metabolism of purine compounds, and both increased urate
production and decreased kidney excretion of uric acid can lead
to hyperuricemia. The American College of Rheumatology
defines hyperuricemia as a serum uric acid concentration of
$6.8 mg/dl (approximately $400
m
mol/l).
607
Data from the US National Health and Nutrition Exami-
nation Survey (NHANES) 2015–2016 found that the crude
adult prevalence of gout (defined as self-reported, doctor
diagnosis, or uric acid–lowering therapy use) was 3.9% with a
higher prevalence in men than women (5.2% vs. 2.7%). After
adjustment for age and sex, an eGFR consistent with CKD G3
was associated with about twice the prevalence of gout (odds
ratio: 1.96; 95% CI: 1.05–3.66).
608
Recommendation 3.14.1: We recommend people
with CKD and symptomatic hyperuricemia should be
offered uric acid–lowering intervention (1C).
The Work Group placed hig h value on avoiding the un-
pleasant symptoms of acute gout and pre venting long-term
complications of recurrent gout among people with CKD.
There are well-tolerated and low-cost oral medications that
can effectively lower blood ur ic ac id concentration in people
with CKD.
Key information
Balance of benefits and harms.
Systematic review of the
management of gout by the American College of Rheu-
matology found strong evidence for uric acid lowering in
people with tophaceous gout, radiographic damage due to
gout, or frequent gout flares; some of whom also had
CKD.
607
The ERT assessed the safe ty of uric acid–lowering therapy
and found that uric acid lowering did not increase adverse
events among people with CKD and particularly focused on
risk of cutaneous reactions and hypersensitivity (pooled RR:
1.00; 95% CI: 0.60–1.65) and hepato toxicity (pooled RR:
0.92; 95% CI: 0.37–2.30). Uric acid–lowering therapy was also
found not to modify the risk of cardiovascular events or all-
cause mortality in people with CKD.
150,609,610
This reassuring
cardiovascular safety profile is consistent with general
population data. In the open -label Allopurinol and
Cardiovascular Outcomes in Patients With Ischemic Heart
Disease (ALL-HEART) randomized trial, 5721 people aged
$60 years with ischemic heart disease but no history of
gout were included. Allopurinol did not modify
cardiovascular risk compared with standard care (HR for
the composite primary outcome of nonfatal myocardial
infarction, nonfatal stroke, or cardiovascular death: 1.04;
95% CI: 0.89–1.21). Findings were similar when 540 people
–20
0
20
40
60
80
100
120
140
160
180
Parathyroid hormone, pg/m
15 30 45 60 75 90 105 120
–0.2
0
0.2
0.4
0.6
0.8
1.0
1.2
15 30 45 60 75 90 105 120
–0.3
–0.2
–0.1
0
0.1
0.2
0.3
Serum calcium (albumin
corrected), mg/d
Serum phosphorus, mg/d
15 30 45 60 75 90 105 120
eGFR, ml/min/1.73 m
2
eGFR, ml/min/1.73 m
2
eGFR, ml/min/1.73 m
2
A1
A2
A3
Figure 35 | Association between estimated glomerular filtration rate (eGFR) with serum concentrations of parathyroid hormone,
phosphate, and serum calcium in general population and high-risk cohorts from the Chronic Kidney Disease Prognosis Consortium, by
level of albuminuria (A1–A3). The y axis represents the meta-analyzed absolute difference from the mean adjusted value at an eGFR of 80 ml/
min per 1.73 m
2
and albumin excretion <30 mg/g (<3 mg/mmol). A1, albuminuria <30 mg/g (<3 mg/mmol); A2, albuminuria 30–300 mg/g
(3–30 mg/mmol); A3, >300 mg/g (>30 mg/mmol). Reproduced from American Journal of Kidney Diseases, volume 73, issue 2, Inker LA, Grams
ME, Levey AS, et al. Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: an individual participant data meta-
analysis in a Global Consortium, pages 206–217, Copyright ª 2018, with permission from the National Kidney Foundation, Inc.
541
chapter 3 www.kidney-international.org
S230
Kidney International (2024) 105 (Suppl 4S), S117–S314

with an eGFR of <60 ml/min per 1.73 m
2
at baseline (among
whom 71 primary outcomes accrued) were compared with
the 5181 people with an eGFR of $60 ml/min per 1.73 m
2
(568 outcomes).
611
Certainty of evidence. The overall certainty of the evidence
for uric acid–lowering therapy among people with CKD and
hyperuricemia is very low (see Supplementary Table
S11
150,612–614
). The critical outcome of delaying progression
of CKD was addressed by 7 RCTs.
150,612,615–619
The 2 largest
RCTs were considered to have a low risk of bias.
615,616
The
certainty of the evidence was downgraded for inconsistency
because there was substantial statistical heterogeneity
detected in the meta-analysis (I
2
¼ 50%) and the estimated
RRs ranged from 0.05 to 2.96. The certainty of the evidence
was further downgraded because of very serious
imprecision. There were 81 kidney failure events among the
participant s in the 7 trials.
The overall certainty of the evidence for delaying pro-
gression is very low, and the certainty for the critical harm
outcomes, such as cutaneous reactions, hypersensitivity, and
hepatotoxicity, was graded as low. However, the certai nty of
evidence for uric acid–lowering interventions in reducing
frequency and severity of gout attack, and limiting tophaceous
deposition is consistently high, so the recommendation is
given an overall grade of level C.
Values and preferences. People with gout have reported
that they were i nitially hesitant to star t ur ic acid –lowering
therapy, but that after experiencing improved cont rol of
inflammator y sy mptoms and tophi, they became strong
advocates for its earlier institution.
607
Resource use and costs. There are several generic xanthine
oxidase inhibitors that are well tolerated and widely available
at low cost.
Considerations for implementation. In most countries, the
cost and availability of uric acid–lowering therapies make
the medications very accessible. The risk of serious adverse
events (e.g., Stevens-Johnson syndrome) is related to the
presence of specific human leukocyte antigen (HLA)
*B5801, which is more common in those of Han Chinese,
Korean, Thai, and African descent. In specific regions,
assessment of the HLA type is recommended before
commencing the drug; where testing is not available, close
monitoring at initiation of the medication should be un-
dertaken. At the current time, there is no indication to
commence medication for high serum ur ic acid levels in
the abs ence of symptoms .
Rationale
Uric acid–lowering therapy reduces ur ic acid levels and their
associated symptomatic joint and skin complications, and are
generally safe to use.
Practice Point 3.14.1: Consider initiating uric acid–lowering
therapy for people with CKD after their first episode of gout
(particularly where there is no avoidable precipitant or serum
uric acid concentration is >9mg/dl[535
m
mol/l]).
Although the initiation of uric acid–lowering therapy in
people with a first gouty arthritis episod e and no tophi was
not recommended by the American College of Rheumatology,
uric acid–lowering therapy use was suggested to be initiated
in people with CKD G3–G5, serum uric acid concentration
>9 mg/dl (>535
m
mol/l), or urolithiasis at the time of their
first episode of gout. This was justified by the higher risk of
gout progression and development of clinical tophi in
CKD.
607
The ERT evidence review identified that uric acid–
lowering therapy results in an increased risk of a gout flare
during the first 3 months after initiation in people with
CKD. This is an expected short-term risk of uric acid
lowering that people should be counseled about when
initiating such therapy. Two relatively small randomi zed
trials have suggested that starting uric acid–lowering
therapy during a gout flare does not appear to extend flare
duration.
620,621
Once initiated, the American College of
Rheumatology suggests continuing uric acid–lowering
therapy indefinitely.
607
Practice Point 3.14.2: Prescribe xanthine oxidase inhibitors
in preference to uricosuric agents in people with CKD and
symptomatic hyperuricemia.
Xanthine oxidase inhibitors (e.g., allopurinol and
febuxostat) reduce serum uric acid concentration by reducin g
purine metabolism into uric acid. Uricosuric agents enhance
its urinary excretion (probenecid is an example), but their
effect is blunted in the context of reduced GFR. Note that the
Cardiovascular Safety of Febuxostat and Allopurinol in Par-
ticipants With Gout and Cardiovascular Comorbidities
(CARES) double-blind randomized trial of allopurinol versus
febuxostat in 6190 people with gout and prior CVD found
that these 2 in terventions were noninferior with respect to the
composite primary cardiovascular outcome. However, overall
mortality and cardiovascular mortality were higher in the
febuxostat group than in the allopurinol group (HR for death
from any cause: 1.22; 95% CI: 1.01–1.47 and HR for car-
diovascular death: 1.34; 95% CI: 1.03–1.73).
622
In people with
T2D, post hoc analyses from 2 large, placebo-controlled RCTs
have reported that SGLT2i reduce serum uric acid
concentration and appeared to reduce gout adverse event
reports or initiations of uric acid–lowering therapy.
515,623
Observational studies suggest that diuretics (thiazide and
loop) increase serum uric acid concentration.
624
The effect
is mediated through multiple potential kidney-centered
mechanisms, which are summarized in a review of drug-
induced hyperuricemia.
625
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S231
Practice Point 3.14.3: For symptomatic treatment of acute
gout in CKD, low-dose colchicine or intra-articular/oral
glucocorticoids are preferable to nonsteroidal anti-inflam-
matory drugs (NSAIDs).
The American College of Rheumatology recommended that
colchicine, NSAIDs, or glucocorticoids are preferred first-line
therapies for acute gout treatment based on demonstrated high
levels of evidence for efficacy, low cost, and tolerability.
607
Administra tion early after symptom onset is encouraged. For
colchicine, the US Food and Drug Administration (FD A)-
approved dosing (1.2 mg immediately followed by 0.6 mg an
hour later, with ongoing anti-inflammatory therapy until the
flare resolves) was highlighted.
607
Dose adjustment should be
consider ed for CKD G5. Anti-inflammatory tr eatment may be
useful as prophylaxis against a symptomatic flare when
initiating uric acid–lowering therapy and may sometimes be
required long term (without diarrhea). We have advised that
low-dose colchicine is preferable to NSAIDs given the safety
and tolerability profile and may also reduce risk of
cardiovascular events.
626
In contrast, NSAIDs can cause toxicity
in CKD and need to be used cautiously.
627
Short courses of
glucocorticoids titrated to symptoms response (e.g., 30 mg
prednisolone orally for 3–5days)couldbeusedasanalternative.
Dietary approaches.
Practice Point 3.14.4: Nonpharmacological interven tions
which may help prevent gout include limiting alcohol,
meats, and high-fructose corn syrup intake.
High alcohol intake, high purine intake, and consumption
of carbonated drinks are associated with higher levels of
serum uric acid. Consumption of these products in higher
amounts is associated with both higher levels and gout
symptoms. In contrast, diets that are low in fat and dairy, and
high fiber, plant-based diets are associated with lower inci-
dence of gout. Thus, diet modification may be of value in
people with CKD, high uric acid, and gout.
Serum uric acid levels among people with a history of gout
are higher in those with higher versus moderate levels of
alcohol intake ($30 units/wk vs. <20 units/wk), as is the risk
of recurrence.
624,628
The odds of gout also appear higher
among those with higher median purine intake ($850 mg
vs. <850 mg estimated purine intake in the last 24 hours).
624
Experimentally, 2 hours after ingestion of 1 g/kg of body
weight of fructose, serum uric acid concentration increases
by 1–2mg/dl(59.5–119
m
mol/l),
629
and its consumption in
carbonated drinks is observationally associated with higher
serum uric acid concentration levels,
630,631
and incident gout
(whereas diet versions of these drinks are not).
632
Foods
associated with a low incidence of gout include low-fat dairy,
and high-fiber and plant-based diets.
633
Special considerations
Pediatric considerations.
There are no uric acid–lowering
trials in children.
International considerations. Asian (as opposed to African
and Caucasian) ethnicities may be at higher risk of serious
skin cutaneous reactions if they carry the HLA-B*5801 allele.
It has been suggested that HLA-B*5801 allele screening may
be considered in people who will be treated with allopurinol
(although there is uncertainty that screening would be cost-
effective).
634
Recommendation 3.14.2: We suggest not using
agents to lower serum uric acid in people with CKD
and asymptomatic hyperuricemia to delay CKD
progression (2D).
The Work Group judged that most well-informed people with
CKD would prefer to optimize medical therapies that have
proven benefit for CKD progression, and that the evidence does
not support treatment of asymptomatic hyperuricemia to modify
risk of CKD progression.
Key information
Balance of benefits and harms.
On balance, despite obse r-
vational studies implicating elevated serum uric acid levels in
the progression of CKD, the data from systematic reviews and
multiple RCTs do not support treatment in the absence of
symptoms. Given the pill burden and lack of data , there is
little support for the use of uric acid–lowering agents.
Observational data that implicate elevated serum uric acid
levels in the progression of CKD have not been shown to
reflect causal associations,
635,636
as RCTs evaluating uric acid
lowering on progression of CKD do not demonstrate clear
benefit on progression, includ ing data summarized in a
Cochrane systematic review comprising 12 RCTs that had
randomized 1187 participants.
609
Since the 2017 Cochrane
review, 3 large, important RCTs with negative results have
been conducted in people w ith CKD and asymptomatic
hyperuricemia (Table 3 0).
615,616,637
The ERT review identified 25 studies (26 publications) that
compared a uric acid–lowering therapy with placebo, usual
care, or another uric acid–lowering therapy among people with
CKD and hyperuricemia.
150,612–619,622,637–652
Twenty-two
studies (23 publications)
150,612–619,622,637–652
were new studies
published since the Cochrane review or were not captured by
the Cochrane 2017 revie w.
609
We did not include 9 studies
from the Sampson et al.
609
review because they did not
include a separate analysis among people with CKD or
because the study was reported as a meeting abstract only.
Among people with CKD and hyperuricemia, the effects of
uric acid–lowering therapy compared with placebo or usual
care were unclear in terms of progression of kidney failure
(pooled RR: 0.92; 95% CI: 0.43–1.98 for studies ranged in
follow-up from 3 months to 7 years), cuta neous reactions
and hypersensitiv ity (pooled RR: 1.00; 95% CI: 0.60–1.65),
and hepatotoxicity (pooled RR: 0.92; 95% CI: 0.37–2.30).
Lastly, within the various therapies among people with CKD
and hyperuricemia, the effects of febuxostat compared with
benzbromarone on cutaneous reactions and hypersensitivity
were unclear (RR: 0.20; 95% CI: 0.01–4.01).
chapter 3 www.kidney-international.org
S232
Kidney International (2024) 105 (Suppl 4S), S117–S314

Certainty of the evidence. The overall certainty of the evi-
dence for uric acid–lowering therapy among people with CKD
and hyperuricemia is very low. The critical outcome of delaying
the progression of CKD was addressed by 7 RCTs.
150,612,615–619
The certainty of the evidence was downgraded for
inconsistency because there was some statistical heterogeneity
detected in our meta-analysis (Supplementary Table S12
615–619,637,638,640,642,651,653
). The certainty of the evidence was
further downgraded because of very serious imprecision, as
there were few events in the trials.
Values and preferences. The Work Group judged that most
well-informed people with CKD would prefer to optimize
medical therapies that have proven benefit for CKD pro-
gression, and that there is little evidence to support the
treatment of asymptomatic hyperuricemia to modify the risk
of CKD progression.
Resource use and costs. There are no cost considerations,
beyond cost-savings, in our recommendation not to use uric
acid–lowering agents.
Considerations for implementation. There are no imple-
mentation considerations in our recommendation not to use
uric acid–lowering agents.
Rationale
There is insufficient evidence to recommend the use of uric
acid–lowering therapies in asymptomatic hyperuricemia for
the specific purpose of delaying CKD progression. We make
the recommendation not g iving uric acid–lowering therapy in
asymptomatic hyperuricemia for slowing of kidney disease
based on the current evidence that suggests unclear benefits.
We judge that it is best practice not to expose people to
medications that provide little benefit.
3.15 Cardiovascular disease (CVD) and additional
specific interventions to modify risk
Prevalence and diagnosis. People with CKD are at increased
risk of CVD,
654,655
a key feature of which is structural heart
disease, heart failure, and sudden death.
656–658
Increased risk
of atherosclerotic disease also accompanies CKD.
654
These
risks increase progressively as eGFR declines (Figure 36
12
).
4
Risk of death from CVD exceeds the risk of progression to
kidney failure for the majority of people with CKD.
The diagnosis of cardiac disease can be more complex and
challenging in CKD, with many standard tests needing careful
Table 30 | Randomized controlled trials in the treatment of asymptomatic hyperuricemia in people with CKD
Study (N) CKD population
Intervention
(follow-up) Outcome
CKD-FIX
615
(N ¼ 369) CKD G3–G4, mean ACR 717 mg/g (81 mg/mmol),
mean urate 8.2 mg/dl (490
m
mol/l)
Allopurinol vs.
placebo (104 wk)
No significant difference in eGFR decline,
3.33 vs. 3.23 ml/min per 1.73 m
2
/yr
PERL Study group
616
(N ¼ 530)
eGFR 40–99.9 ml/min per 1.73 m
2
and type 1 diabetes
Allopurinol vs.
placebo (3 yr)
No significant difference in mGFR decline,
3.0 vs. 2.5 ml/min per 1.73 m
2
/yr
FEATHER Study
637
(N ¼ 467)
CKD G3 Febuxostat vs.
placebo (108 wk)
No significant difference in eGFR slope
0.23 5.26 vs. 0.47 4.4.8 ml/min per
1.73 m
2
ACR, albumin-to-creatinine ratio; CKD, chronic kidney disease; CKD-FIX, Controlled Trial of Slowing of Kidney Disease Progression from the Inhibition of Xanthine Oxidase;
eGFR, estimated glomerular filtration rate; FEATHER, Febuxostat vs. Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients With Hyperuricemia
Complicated by Chronic Kidney Disease Stage 3; PERL, Preventing Early Renal Loss in Diabetes.
105+
90–104
60–89
45–59
30–44
15–29
<15
<10 10–29 30–299 300–999 1000+
Overall
eGFRcr
ACR (mg/g)
All-cause mortality: 82 cohorts
Study size = 26,444,384; events = 2,604,028
105+
90–104
60–89
45–59
30–44
15–29
<15
<10 10–29 30–299 300–999 1000+
Overall
eGFRcr
ACR (mg/g)
Cardiovascular mortality: 76 cohorts
Study size = 26,022,346; events = 776,441
1.6 2.2 2.9 4.3 5.8
Ref 1.3 1.8 2.6 3.1
1.0 1.3 1.7 2.2 2.8
1.3 1.6 2.0 2.4 3.1
1.8 2.0 2.5 3.2 3.9
2.8 2.8 3.3 4.1 5.6
4.6 5.0 5.3 6.0 7.0
1.4 2.0 3.0 4.1 5.4
Ref 1.3 1.9 2.7 3.6
1.0 1.4 1.7 2.4 3.2
1.4 1.7 2.2 2.8 3.8
2.0 2.3 2.8 3.7 4.6
3.2 3.1 3.5 5.0 6.5
6.1 6.4 6.4 7.3 8.2
Figure 36 | Risk of all-cause and cardiovascular mortality by estimated glomerular filtration rate (eGFR) and level of albuminuria from
general population cohorts contributing to the Chronic Kidney Disease Prognosis Consortium. ACR, albumin-to-creatinine ratio; eGFRcr,
creatinine-based estimated glomerular filtration rate. Reproduced with permission from JAMA, Writing Group for the CKD Prognosis
Consortium; Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration rate, albuminuria, and adverse outcomes: an individual-
participant data meta-analysis. JAMA. 2023;330(13):1266– 1277.
12
Copyright ª 2023 American Medical Association. All rights reserved.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S233

consideration in people with CKD.
659,660
For example, exercise
electrocardiography may be limited through inability to
exercise to a diagnostic workload, or presence of microvascular
disease. Perceived risks of contrast agents may limit the use of
diagnostic imaging, thus impacting treatment choices; the risks
of contrast agents may limit the use of imaging. In addition, a
strain pattern may mask diagnostic ST depression, and acute
coronary syndrome is less likely to present with classical
ischemic symptoms and electrocardiographic changes than in
the general population, instead often manifesting as heart
failure symptoms or syncope.
659,660
In people with GFR <60
ml/min per 1.73 m
2
(GFR categories G3a–G5), KDIGO has
previously recommended that serum concentrations of
troponin be interpreted with caution with respect to diagnosis
of acute coronary syndrome.
1
More sensitive troponin assays
maintain high diagnostic accuracy in people with CKD, but
higher assay-specific optimal cutoff levels may be
considered.
661
Regardless of assay, careful attention to trends in
troponin concentration over time is required through serial
measurement.
662
Management. In people with CKD, the same principles
should be used to manage atherosclerotic risk as in people
without CKD. The level of care for CVD offered to people
with CKD should not be prejudiced by their GFR. Data
suggest the underuse of proven effective treatment in
people with CKD presenting with acute coronary
syndrome.
663
Prevention of ASCVD should consider pharmaceutical,
dietary, and lifestyle intervention, which target traditional
cardiovascular risk factors (e.g., BP and dyslipidemias), as
well as CKD-MBD, which accelerates vascular calcification
resulting in both vascular intima (resulting in increased
amounts of calcium in atherosclerotic plaques
664
) and
vascular media calcification (leading to increased vascular
stiffness).
604
3.15.1 Lipid management
Dyslipidemia in CKD is frequently characterized by high tri-
glycerides, low HDL cholesterol, and an increased proportion
of low-density lipoprotein (LDL) particles, which are small and
oxidized.
665
In adults with newly identified CKD, it has been
recommended to evaluate their lipid profile (total cholesterol,
LDL cholesterol, HDL cholesterol, and triglycerides), but
follow-up lipid measurements are not required for the
majority of people (i.e., a fire-and-forget policy is
recommended).
19
This is because treatment initiation is
based on risk, and the benefits of statin-based therapy have
been shown to be independent of the level of cholesterol. For
those with a total cholesterol >7.5 mmol/l (290 mg/dl) and
a personal or family history of premature ischemic heart
disease (e.g., an event before the age of 60 years in an
individual or first-degree relative), it is important to consider
familial disease and specialist referral.
666
The benefits of lowering LDL cholesterol using statin-
based therapies on the risk of ASCVD are well established in
people with and without CKD. There are clear
recommendations on when to initiate such therapies set out
in the KDIGO Clinical Practice Guideline for Lipid
Management in Chronic Kidney Disease.
19
The Work
Group concurs with all the recommendations in this
guideline. In particular, we draw attention to:
Recommendation 3.15.1.1: In adults aged ‡50 years
with eGFR <60 ml/min per 1.73 m
2
but not treated
with chronic dialysis or kidney transplantation (GFR
categories G3a–G5), we recommend treatment with
a statin or statin/ezetimibe combination (1A).
Recommendation 3.15.1.2: In adults aged ‡50 years
with CKD and eGFR ‡60 ml/min per 1.73 m
2
(GFR
categories G1–G2), we recommend treatment with a
statin (1B).
Recommendation 3.15.1.3: In adults aged 18–49 years
with CKD but not treated with chronic dialysis or kid-
ney transplantation, we suggest statin treatment in
people with one or more of the following (2A):
known coronary disease (myocardial infarction or
coronary revascularization),
diabetes mellitus,
prior ischemic stroke, or
estimated 10-year incidence of coronary death or
nonfatal myocardial infarction >10%.
The Work Group offers the following practice points to
support implementation of the recommendations above.
Practice Point 3.15.1.1 Estimate 10-year cardiovascular risk
using a validated risk tool.
Details of the Work Group recommendations on how to
estimate risk are provided in Chapter 2, Section 2.3.Currently,
the CKD patch for the Systematic Coronary Risk Evaluation
(SCORE) tool and the American Heart Association
PREVENT
,
pending
equations are the only ones validated.
Practice Point 3.15.1.2: In people with CKD, choose statin-
based regimens to maximize the absolute reduction in low-
density lipoprotein (LDL) cholesterol to achieve the largest
treatment benefits.
Since 2013, published literature has continued to demon-
strate the general safety of statin-based therapies.
667
This
includes individual participant-level data meta-analysis by the
Cholesterol Treatment Trialists’ collaboration, showing that
statin therapy causes only a small excess of mild muscle pain,
and most (>90%) of all reports of muscle symptoms among
users are not due to their statins.
668
In CKD, the Study of
Heart and Renal Protection (SHARP) demonstrated that an
intensive statin-based regimen was safe and not associated
with any serious nonvascular hazard.
669,670
A Cholesterol
Treatment Trialists’ collaboration meta-analysis combining
SHARP with the other large trials took into account the
smaller reductions in LDL cholesterol achieved with statin-
based therapy in people with CKD G3–G5. After
chapter 3 www.kidney-international.org
S234
Kidney International (2024) 105 (Suppl 4S), S117–S314

standardization to a 1.0 mmol/l (38.7 mg/dl) LDL cholesterol
difference, the RR reductions in major vascular events
observed w ith statin-based treatment in the large statin trials
were shown to become progressively smaller as eGFR
declines, with little evidence of benefit in people on dialysis
(Figure 37 ).
671
The corollary of this obser vation is that in
people with CKD, statin-based regimens should be chosen to
maximize the absolute reduction in LDL cholesterol to
achieve the largest treatment benefits. Large trials have shown
the following once-daily intensive statin-based regimens are
safe in CKD (including people on dialysis): atorvastatin
20 mg,
672
rosuvastatin 10 mg,
673
and simvastatin 20 mg
combined with ezetimibe 10 mg.
669,670
Practice Point 3.15.1.3: In adults with CKD aged 18–49, a
lower (i.e., <10%) esti mated 10-year incidence of coronary
death or nonfatal myocardial infarction may also be
appropriate thresholds for initiation of statin-based
therapy.
The Work Group deems it appropriate to consider lower
thresholds for the initiation of statin-based therapy in adults
with CKD than suggested in the KDIGO 2013
Major coronary event
eGFR ≥60 m
min per 1.73 m
2
eGFR 45 to <60 m /min per 1.73 m
2
eGFR 30 to <45 m /min per 1.73 m
2
eGFR <30 m /min per 1.73 m
2
not on dialysis
On dialysis
Total
Coronary revascularisation
eGFR ≥60 m
/min per 1.73 m
2
eGFR 45 to <60 m /min per 1.73 m
2
eGFR 30 to <45 m /min per 1.73 m
2
eGFR <30 m /min per 1.73 m
2
not on dialysis
On dialysis
Total
Stroke
eGFR ≥60 m
/min per 1.73 m
2
eGFR 45 to <60 m /min per 1.73 m
2
eGF 30 to <45 m /min per 1.73 m
2
eGFR <30 m min per 1.73 m
2
not on dialysis
On dialysis
Total
Major vascular event
eGFR ≥60 m
/min per 1.73 m
2
eGFR 45 to <60 m /min per 1.73 m
2
eGFR 30 to <45 m /min per 1.73 m
2
eGFR <30 m /min per 1.73 m
2
not on dialysis
On dialysis
Total
RR (CI) per 1.0 mmol/
reduction in LDL
cholesterol
P for
trend
Statin or more
intensive regimen
Control or less
intensive regimen
Number of events (% per annum)
3200 (1.2%)
1157 (1.7%)
457 (2.3%)
163 (1.5%)
264 (2.1%)
5303 (1.4%)
3943 (1.5%)
1039 (1.5%)
265 (1.3%)
99 (0.9%)
183 (1.5%)
5618 (1.5%)
1408 (0.5%)
575 (0.8%)
263 (1.3%)
116 (1.1%)
213 (1.7%)
2591 (0.7%)
7348 (2.9%)
2377 (3.6%)
863 (4.5%)
320 (3.0%)
571 (4.7%)
11,617 (3.2%)
4178 (1.6%)
1479 (2.2%)
567 (2.8%)
179 (1.7%)
287 (2.3%)
6761 (1.8%)
4963 (1.9%)
1387 (2.1%)
328 (1.6%)
123 (1.2%)
224 (1.8%)
7113 (1.9%)
1661 (0.6%)
708 (1.0%)
284 (1.4%)
137 (1.3%)
199 (1.6%)
3019 (0.8%)
8933 (3.6%)
3013 (4.6%)
1014 (5.2%)
364 (3.5%)
599 (5.0%)
14,079 (3.9%)
0.74 (0.70–0.79)
0.76 (0.69–0.84)
0.80 (0.68–0.95)
0.87 (0.68–1.12)
0.89 (0.70–1.14)
0.76 (0.73−0.79)
0.76 (0.71–0.80)
0.71 (0.64–0.80)
0.81 (0.64–1.02)
0.78 (0.57–1.05)
0.78 (0.58–1.05)
0.75 (0.73−0.78)
0.83 (0.76–0.92)
0.81 (0.70–0.93)
0.91 (0.73–1.13)
0.83 (0.63–1.10)
1.09 (0.82–1.44)
0.84 (0.80−0.89)
0.78 (0.75–0.82)
0.76 (0.70–0.81)
0.85 (0.75–0.96)
0.85 (0.71–1.02)
0.94 (0.79–1.11)
0.79 (0.77−0.81)
0.01
0.9
0.07
0.008
LDL cholesterol
lowering worse
LDL cholesterol
lowering better
99% or 95% CI
0.750.5 1.00 1.50
Figure 37 | Effect of lowering low-density lipoprotein (LDL) cholesterol per 1.0 mmol/l on risk of major vascular events by level of
estimated glomerular filtration rate (eGFR) at recruitment. Meta-analysis of 28 large trials of statin-based therapy using individual
participant level data. The black squares and horizontal lines represent 99% confidence intervals (CIs), with diamonds representing 95% CI. RR,
relative risk. Reproduced from Herrington WG, Emberson J, Mihaylova B, et al. Impact of renal function on the effects of LDL cholesterol
lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol.
2016;4:829–839.
671
ª The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY license.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S235

recommendations. There is good evidence for the safety of
intensive LDL-cholesterol lowering, and statin-based the rapy
combined with a fire-and-forget strategy is low cost. This
approach is consistent with a more recent recommendation
for primary prevention in CKD by the American College of
Cardiology/American Heart Association (which recom-
mended 10-year thresholds of >7.5%).
674
Practice Point 3.15.1.4: Consider prescribing proprotein
convertase subtilisin/kexin type 9 (PCSK-9) inhibitors to
people wit h CKD who have an indication for their use.
Proprotein convertase subtilisin/kexin type 9 (PCSK-9) in-
hibitors have been shown to safely reduce ASCVD risk when
added to maximal tolerated statin-based regimens in people at
high coronary risk.
675,676
Subgroup analyses suggest that their
safety profile and their biochemical and clinical efficacy are
similar when participants with CKD and without CKD are
compared. These trials recruited down to an eGFR of 20 ml/
min per 1.73 m
2
.
677,678
Current examples of recommendations
for the use of PCSK-9 inhibitors from the cardiology
community (and lic ensed indications) include as an adjunct to
diet and maximally tolerat ed statin therapy for the treatment of
adults with heterozygous familial hypercholesterolemia or for
people with clinical ASCVD who require additional lowering of
LDL cholesterol.
679,680
Dietary approaches.
Practice Point 3.15.1.5: Consider a plant-based “Mediter-
ranean-style” diet in addition to lipid-modifying therapy to
reduce cardiovascular risk.
Diet and lipids have been comprehensively reviewed by
other clinical practice guidelines.
679,681
In that work, the
Work Groups highlighted that in general populations,
observational studies have associated plant-based diets that
include higher consumption of fruit, vegetables, nuts,
legumes, fish, olive oil, yogurt, and w hole grains, with lower
risk of CVD. Diets associated with higher risk are those
including high consumption of red and processed meats,
refined car bohydrates, and salt. Vegetable sources of fats
and polyunsaturated fatt y acids (e.g., in nuts, seeds,
avocado, and olive oil) are also associated with a lower risk
compared with animal fats, including dairy fat.
679
A
Mediterranean-style diet has an emphasis on extra virgin
olive oil and is high in unsaturated fat. RCTs have shown
that such diets have imp ortant effects on cardiovascular risk
in the long term despite only small effects on traditional
markers of metabolic syndrome profile.
682–685
In the large
Prevención con Dieta Mediterránea (PREDI MED) primary
prevention trial of 7447 adults, the Mediterranean diet rich
in extra virgin olive oil reduced the risk of major
cardiovascular events by 31% (HR: 0.69; 95% CI: 0.53–
0.91). The Coronary Diet Intervention With Olive Oil and
Cardiovascular Prevention (CORDIOPREV) trial found that
allocation to a Mediterranean diet rich in extra virg in olive
oil reduced the risk of the composite of MACE by
approximately 22%–25%.
684
There is no large-scale CKD-
specific trial comparing these dietary interventions.
3.15.2 Use of antiplatelet therapy
Recommendation 3.15.2.1: We recommend oral low-
dose aspirin for prevention of recurrent ischemic
cardiovascular disease events (i.e., secondary pre-
vention) in people with CKD and established
ischemic cardiovascular disease (1C).
This recommendation places high value on the importance of
reducing recur rence of myocardial infarction, ischemic strokes,
or peripheral arter ial disease complications in people with
CKD and established ischemic CVD due to the mortality and
disability associated w ith such complications. In secondar y
prevention, t rials have clearly shown the absolute benefits of
low-dose aspir in substantially exceed the potential for bleeding
complications, creating certainty about net benefits when
treating this population. In people w ith CKD without prior
ischemic CVD, the balance of benefits and risks are uncertain
and may be counterbalanced—large RCTs are ongoing.
Key information
Balance of benefits and harm.
Based on a number of large
RCTs in populations that are likely to be largely free from
CKD, lifelong use of low-dose aspirin (75–100 mg) for the
prevention of recurrence of complications of ischemic CVD is
strongly recommended among people with known CVD (a
therapeutic approach referred to as secondary prevention).
Conversely, it is not possible to provide definitive recom-
mendations on when to use aspirin to prevent a first ischemic
cardiovascular event (i.e., primary prevention) in people at
high risk, and a research recommendation is provided. This is
due to uncertainty of the net absolute value of such an
approach, as any reduction in the risk of atherosclerotic
cardiovascular events needs to be weig hed against the risk of
major bleeding. It is imp ortant to consider CKD -specific data
in the totality of the evidence.
Key evidence from general populations is derived from a
2009 meta-analysis by the Anti-thrombotic Treatment Tria-
lists’ collaboration. The analyses included data on long-term
aspirin use versus control care in 16 secondary prevention
trials (approximately 17,000 people at high average risk,
approximately 43,000 person-years, 3306 serious vascular
events [defined as myocardial infarction, stroke, or cardio-
vascular death]), and 6 primary prevention trials (approxi-
mately 95,000 participants at low average risk, approximately
660,000 person-years, 3554 serious vascular events).
685a
In the
secondary prevention trials, allocation to aspirin reduced the
risk of both ischemic stroke and myocardial infarction by
about one-fifth, such that an overall RR reduction for any
serious vascular event was 19% compared with controls
(RR: 0.81; 95% CI: 0.75–0.87). This equated to a 1.49%
chapter 3 www.kidney-international.org
S236
Kidney International (2024) 105 (Suppl 4S), S117–S314

lower absolute risk of serious vascular events per year
compared with an estimated absolute risk of any major
bleeding, which was an order of magnitude smaller at
0.03% per year. Note that this hazard of major bleeding was
extrapolated from the primary prevention trials as stroke
causes and extracranial bleeds were generally not well
recorded in the relatively older secondary prevention trials
(Figure 38
685a
).
Some people with CKD have been included in antiplatelet
therapy trials. A recent Cochrane collaboration meta-analysis
of 40,597 trial participants with CKD recruited into anti-
platelet versus placebo trials and 11,805 recruited into anti-
platelet agent comparison trials found that allocation to
antiplatelet therapy may reduce the RR of myocardial
infarction by approximately 12% (RR: 0.88; 95% CI: 0.79–
0.99). There was an expected increased risk of major bleeding,
Primary
0
10
20
30
40
50
60
0
10
20
30
40
50
60
Secondary
0.3% 0.2%
0.9%
21.0%
25.9%
0.9%
0.5%
3.9%
4.5%
38.7%
45.2%
1.2%
AC A C
AC
AC
AC
AC
AC
AC
AC
AC
AC
AC
0.7%
8.0%
0.5%
Male, entry age 50–59 years
Female, entry age 50–59 years Female, entry age 65–74 years
Male, entry age 65–74 years
0.3%
3.4%
3.9%
29.7%
36.7%
5-year risk (%) 5-year risk (%)
9.2%
55.8%
47.7%
1.1%
Primary Secondary
Vascular death
Nonfatal MI/stroke
Nonfatal GI or other
extracranial bleed
Figure 38 | Predicted 5-year absolute benefits and harms of allocation to aspirin (A) versus control (C) using a secondary or primary
prevention strategy, by different levels of risk (based on age and sex). GI, gastrointestinal; MI, myocardial infarction. Reproduced with
permission from The Lancet, volume 373, Antithrombotic Trialists' (ATT) Collaboration, Aspirin in the primary and secondary prevention of
vascular disease: collaborative meta-analysis of individual participant data from randomised trials, pages 1849–1860, Copyright ª 2009 Elsevier
Ltd.
685a
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S237

but the magnitude of the RR was consistent with the data
from general populations (RR: 1.35; 95% CI: 1.10–1.65).
686
Note that these analyses did not distinguish between
primary and secondary prevention settings.
686
The 2009
Anti-thrombotic Treatment Trialists’ collaboration meta-
analysis and results from 3 more recent large tr ials (A Study
of Cardiovascular Events in Diabetes [ASCEND],
687
Aspirin
in Reducing Events in the Elderly [ASPREE],
688
and Aspirin
to Reduce Risk of Initial Vascular Events [ARRIVE]
689
)
assessing the effects of aspirin versus placebo for primary
prevention in specific high-risk populations found that any
harm from major bleeding counterbalanced any benefitof
aspirin on cardiovascular risk (with ASPREE and ARRIVE
both finding no significant effect on cardiovascular events
in their studied populati ons of older adults or high-risk
adults, respectively).
685a
A dedicated large primary
prevention aspirin trial in CKD is underway.
690
Certainty of evidence. The 2009 meta-analysis by the Anti-
thrombotic Treatment Trialists’ collaboration on the effect of
aspirin compared with placebo in terms of the primary and
secondary prevention of CVD and safety among people with
and without CKD was assessed to have high risk of bias using
the Risk of Bias Asses sment Tool for Systematic Reviews
(ROBIS) checklist due to unclear identification and selection
of studies, unclear data collection and study appraisal, and
high risk of bias for synthesis and findings (although we did
not contact the authors to clarify these details).
685a
This
review did not report on the evidence or certainty of
evidence assessments directly in the report. Given the
available evidence, the recommendation has a low certainty
of ev idence (Level C).
Values and preferences. Maintaining QoL by minimizing
risk of worsening of ischemic heart disease and recurrent
stroke-related disability is important to both people w ith
CKD and caregivers.
691
The Work Group considered that the
risk of bleeding would be considered acceptable by most
people with CKD once the clear net benefits were explained
and gastroprotection was offered. The Work Group
considered that some people with CKD without prior
ischemic coronary, cerebrovascular, or peripheral arterial
disease but at increased risk (e.g., due to diabetes) may still
wish to consider using aspirin and accept the risk of major
bleeding.
687
Some people with CKD may also have a kidney
diagnosis that indirectly supports considering the use of
aspirin despite a lack of evidence (e.g., presumed or proven
renovascular disease). The Work Group is not aware of any
risk tools that could be used to help counsel such pe ople
with CKD as to their expected net absolute benefits and
risks based on r isk factors of the person with CKD,
including any difference by sex. (Note that scores to predict
cardiovascular risk are considered in Chapter 2.)
Resource use and costs. Low-dose aspirin is available at low
cost and does not require monitoring.
Considerations for implementation. Proton-pump inhibitors
(PPIs) are generally effective,
692
safe, and low cost (although
occasionally associated with an interstitial nephritis), and
the Work Group considers that it is prudent to consider
bleeding risk and offers PPIs when prescrib ing antiplatelet
therapy or antithrombotic therapy, particularly when such
therapies are combined.
693
Rationale
Meta-analysis of trials h as clearly established the cardio-
vascular benefits of low-dose aspirin in people who have
established ASCVD. Any harm of bleeding is far outweighed
by the b e n e fits (unlike the situation for primar y prevention,
where bleeding risk has been consistently i dentified in
large aspirin trials and cardiovascular benefits to date have
not).
Practice Point 3.15.2.1: Consider other antiplatelet therapy
(e.g., P2Y
12
inhibitors) when there is aspirin intolerance.
Bleeding from gastrointestinal mucosa with antiplatelet
therapy is likely to be due to their effect on hemostasis of pre-
existing mucosal lesions. This hypothesis is supported by
P2Y
12
inhibitors (e.g., clopidogrel or ticagrelor) not reducing
the risk of bleeding in trials comparing them to aspirin.
694,695
However, if people are aspirin intolerant, a P2Y
12
inhibitor is
a noninferior alternative. Note that in 2009, the FDA
recommended that the coadministration of clopidogrel and
omeprazole (a PPI) should be avoided because omeprazole
reduces the effectiveness of clopidogrel. There is uncertainty
about the precise effect of omeprazole as pharmacokinetic
data are inconclusive, but PPIs with inhibition of CYP2C19
are preferred when using clopidogrel.
696
Guidelines from the cardiology community provide rec-
ommendations for the use of dual antiplatelet therapy for a
period after acute coronary syndrom e or percutaneous
coronary inte rvention. These guidelines recommend to
apply the same diagnostic and therape utic strateg ie s in
people with CKD.
663
CKD does not modif y the benefits of
ticagrelor,
697
and antiplatelet therapy doses do not need to
be mod ified at decreased eGFR. Note that other
antithrombotic therapy choices and doses may need to
consider a person’sGFR.
Special considerations
International considerations.
Given the clinical effectiveness
of low-dose aspirin and its low cost, there should not be many
barriers to accessing this medication in any setting.
3.15.3 Invasive versus intensive medical therapy for coronary
artery disease
Recommendation 3.15.3.1: We suggest that in stable
stress-test confirmed ischemic heart disease, an
initial conservative approach using inte nsive medi-
cal therapy is an appropriate alternative to an initial
invasive strategy (2D).
chapter 3 www.kidney-international.org
S238
Kidney International (2024) 105 (Suppl 4S), S117–S314

This recommendation places high value on the finding from
recent, large trials in both general and CKD populations that
have suggested that intensive medical therapy is a suitable initia l
strategy for the management of stable stress-test confirmed
ischemic heart disease. It places value on the need for in-
terventions, which carry risk to people with CKD and sub-
stantial healthcare costs to demonstrate benefits on
cardiovascular outcomes before they are considered a standard of
care. Importantly, this recommendation should not apply to
those with severe angina symptoms, left ventricular dysfunction
(e.g., ejection fraction <35%), or left main stem disease as they
were excluded from the definitive trials. It should be noted that
trials in CKD have not ruled out antianginal benefits in people
with CKD (despite negative findings).
Key information
Balance of benefits and harm. Benefits.
Benefits should be
considered in the context of the totality of evidence in people
with and without CKD regarding interventions. Comparisons
between aggressive medical therapy alone and invasive in-
terventions do not support invasive strategies to reduce death
or prevent myocardial infarction.
707,707a
However, those with
frequent angina symptoms (at least weekly) gained
improvement with the invasive strategy
707
; thus, the benefit
of an invasive strategy might be restricted to those with
angina. The reason for a lack of clear antianginal effect of an
invasive strategy in International Study of Comparative
Health Effectiveness w ith Medical and Invasive Approaches—
Chronic Kidney Disease (ISCHEMIA-CKD) needs some
consideration, and key reasons relating to insufficient power
due to protocol differences have been proposed.
698
Although
low power to detect an effect on angina is a key potential
explanation for differences in findings between the 2 trials,
CKD-MBD and coronary calcification in CKD, which makes
microvascular disease more common and increases the
technical challenge of revascularization, may also have partly
contributed to these differences.
699
The ERT assessed the effects of angiography or coronary
intervention in people with CKD and ischemic heart disease
identified 4 other trials, but excluded mixed populati ons,
including ISCHEMIA-CKD, which recruited some people on
dialysis and some people who have received a kidney trans-
plant. The review found no clear benefits on cardiovascular
outcomes in 3 other trials and raised a hypothesis about
beneficial effects on mortality overall (Supplementary Table
S13
700–704
). Such an effect has not been observed in the
larger general population trials.
Harms. The harms of invasive strategies include the risk of
dialysis initiation, death, and stroke risk (stroke was inter-
estingly not periprocedural).
707
Certainty of evidence. The ERT review was limited to trials
only recruiting people with CKD (and did not include the
ISCHEMIA-CKD trial discussed above due to the inclusion of
some people on dialysis and some people who have received a
kidney transplant). The overall certainty of the evidence
comparing coronary revascularization with optimal medical
therapy among people with CKD not undergoing KRT and
ischemic heart disease is very low (Supplementary Table
S13
700–704
). Most of the RCTs reporting on the critical
outcomes (all-cause mortality, CVD mortality, CVD events,
kidney failure, and AKI) had some concerns regarding the
risk of bias, particularly with lack of blinding for the
outcome assessors, participants crossing over to the other
treatment group, and the selection of reporting. The
certainty of the evidence was downgraded for all outcomes
because of imprecision. The certainty of the evidence for
cardiovascular mortality was downgraded because
publication bias was strongly suspected.
Values and preferences. Although this was not confirmed
by ISCHEMIA-CKD, antianginal benefits of an invasive
strategy are apparent in general populations, and people with
symptoms may still elect for an initially invasive approach to
manage stable stress-test confirmed coronary artery disease
after being counseled about the risks.
Resource use and costs. It is not possible to formally assess
the cost-effectiveness of intensive medical therapy versus an
initial invasive strategy due to mixed findings from the ev i-
dence in people with stable ischemic heart disease. However,
invasive strategies will have higher cost implications to
healthcare systems, people with CKD, or both.
Considerations for implementation. Access and availability
of inv asive therapies will vary in different healthcare sys-
tems, as might the availability o f medications for maximal
medical therapy. The key to implementation is to encourage
the understanding of the value of full therapy as compared
with invasive therapy so that healthcare providers and
people w ith CKD understand the risks and benefits of
invasive strategies. Given the costs of invasive strategies,
there may be additional value to impleme nting this
recommendation.
Rationale
Evidence suggests that the key indication for an initial invasive
strategy to manage stable ischemic heart disease is based on
symptoms, and intensive medical therapy is a suitable
approach if symptom control is satisfactory in people with or
without CKD. In CKD, the antianginal benefits of an initially
invasive approach have not been demonstrated.
Practice Point 3.15.3.1: Initial management with an inva-
sive strategy may still be preferable for people with CKD
with acute or unstable coronary disease, unacceptable levels
of angina (e.g., patient dissatisfaction), left ventricular
systolic dysfunction attributable to ischemia, or left main
disease.
The ISCHEMIA trial has been described as deeply dis-
rupting prior attitudes regarding management strategies for
people with stable coronary artery disease,
705
and clinical
practice guidelines that predate the trial need updating.
706
Despite the International Study of Comparative Health
Effectiveness with Medical and Invasive Approaches
(ISCHEMIA) and ISCHEMIA-CKD trial results, it is
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S239

considered that the well-established intervention of coronary
revascularization will continue to have a key role in angina
relief.
705
Importantly, this recommendation should not
apply to those with unacceptably severe angina symptoms.
It should also be noted that people with left ventricular
dysfunction (i.e., ejection fraction <35%) or left main
disease were excluded from the defini tive ISCHEMIA
trial.
707a
The Work Group considers that certain design
features of the ISCHEMIA-CKD trial may have led to
angina benefits not being detected, and the trial results
should not rule out angina benefits in people with CKD
(see above). If an invasive strategy is pursued, there are
effective strategies to reduce the risk of contrast-induced
AKI (Chapter 4).
708
The totality of the evidence from the CKD-specific trials is
consistent with no net difference between an initial conser-
vative approach using aggressive medical therapy versus an
invasive strategy when treating stable stress-test confirmed
ischemic heart disease. This is consistent with the large gen-
eral populati on-based ISCHEMIA trial.
707a
3.16 CKD and atrial fibrillation
In CKD, the same principles to diagnose and manage atrial
fibrillation should be used as in people without CKD.
Prevalence and consequences. Atrial fibrillation is the com-
monest sustained arrhythmia, with risk increasing steeply with
increasing age (earlier in men than women).
709
There is a
particularly high prevalence in people with CKD. Crude
prevalence ranging from 16% to 21% has been reported in
people with CKD not requiring KRT.
710
In the cohorts
contributing to the CKD-PC, adults with CKD G3, A1 had
an adjusted risk of atrial fibrillation of 1.2–1.5, incr easing to
an adjusted risk of 4.2 by CKD stages G5, A3 (Figure 39
12
).
Atrial fibrillation can directly cause thromboembolism
(particularly stroke) and/or heart failure. It is also linked,
perhaps directly or through shared risk factors, with increased
risk of death, hospitalization, vascular dementia, depression,
and reduced QoL.
709
Detailed clinical practice guidelines have
been formulated by the cardiology community describing
definitions, classifica tion, diagnosis, screening strategies, and
management.
709
It is beyond the scope of this KDIGO
guideline to consider all aspects of the diagnosis and
management of atrial fibrillation in people with CKD . The
ER T review focused on the role of non–vitamin K antagonist
oral anticoagulants (NOACs) versus warfarin for
thromboprophylaxis in CKD .
Identification and management. Atrial fibrillation can be
asymptomatic but symptoms are not a prerequisite for risk of
complications. As the prevalence of atrial fibrillation is high in
people with CKD and there are effective strategies to manage
its associated complications, opportunistic pulse-based
screening (e.g., when taking BP), followed by a 12-lead
electrocardiogram if an irregularly irregular pulse is identified
should be considered. Such an approach is low cost and
simple to implement. Figure 40 outlines approaches to
different diagnostic and management strategies.
Practice Point 3.16.1: Follow established strategies for the
diagnosis and management of atrial fibrillation (Figure 40).
Prophylaxis against stroke and systemic thromboembolism.
Recent cardiology guidelines recommend a risk factor–based
approach to stroke thromboprophylaxis decisions in atrial
fibrillation using the Congestive heart failure, Hypertension,
Age $75 (doubled), Diabetes, Stroke (doubled), Vascular
disease, Age 65 to 74, and Sex category (female) (CHA
2
DS
2
-
VASc) stroke risk score. They recommend that only people at
“low stroke risk” (CHA
2
DS
2
-VASc score ¼ 0inmen,or1in
women) should not be offered antithrombotic therapy. Oral
anticoagulants should be considered for stroke prevention
with a CHA
2
DS
2
-VASc score of 1 in men or 2 in women,
considering net clinical benefit and values and preferences
of peop le with CKD. Oral anticoagulants are clearly recom-
mended for stroke prevention in people with atrial fibrillation
and a CHA
2
DS
2
-VASc score $2 in men or $3 in women.
709
Our Work Group considered that oral anticoagulation for
thromboprophylaxis should nearly always be considered for
preventing stroke in people with decreased eGFR and atrial
fibrillation (Figure 40). The presence of decreased GFR is a
risk for thromboembolic stroke in people with atrial
fibrillation.
710–712
It has been estimated that approximately
95% of people with an eGFR of <60 ml/min per 1.73 m
2
have a CHA
2
DS
2
-VASc score of $2, increasing to
approximately 99% at an eG FR of <30 ml/min per 1.73
m
2
.
711
Importantly, it has also been shown that in a group
of people with a CHA
2
DS
2
-VASc score of 0 to 1 point (i.e.,
a group where thromboprophylaxis may not be considered
indicated), people with CKD within the group are at much
higher risk of cerebrovascular and other systemic
105+
90–104
60–89
45–59
30–44
15–29
<15
<10 10–29 30–299 300–999 1000+
1.1 1.3 1.7 2.4 3.5
Ref 1.2 1.5 1.9 2.3
1.0 1.2 1.4 1.7 2.2
1.2 1.3 1.5 1.8 2.4
1.4 1.5 1.7 2.0 2.4
1.9 1.8 2.0 2.6 3.0
2.6 2.5 3.1 3.6 4.2
Overall
eGFRcr
ACR (mg/g)
Atrial brillation: 50 cohorts
Study size = 22,886,642; events = 1,068,701
Figure 39 | Meta-analyzed adjusted prevalence of atrial
fibrillation from cohorts contributing to the Chronic Kidney
Disease Prognosis Consortium, by diabetes status. ACR, albumin-
to-creatine ratio eGFRcr, creatinine-based estimate glomerular
filtration rate. Reproduced with permission from JAMA, Writing Group
for the CKD Prognosis Consortium; Grams ME, Coresh J, Matsushita K,
et al. Estimated glomerular filtration rate, albuminuria, and adverse
outcomes: an individual-participant data meta-analysis. JAMA.
2023;330(13):1266–1277.
12
Copyright ª 2023 American Medical
Association. All rights reserved.
chapter 3 www.kidney-international.org
S240
Kidney International (2024) 105 (Suppl 4S), S117–S314

thromboembolic events, with an annual rate of 2.9%
compared with 0.2% in people without CKD.
711
Including GFR into atrial fibrillation risk scores has not
shown important incremental benefit to its introduction (e.g.,
adding 2 points for CrCl <60 ml/min to CHADS
2
—referred
to as Renal Dysfunction, Congestive Heart Failure, Hyper-
tension, Age, Diabetes, Stroke/Transient Ischemic Attack
[R
2
CHADS
2
])—improved net reclassification index but not
the C-statistic.
710
However, as decreased GFR is associated
with age, diabetes, CVD, and so on, the incremental
predictive advantage by adding a CKD parameter to the
CHA
2
DS
2
-VASc score, which already includes these
parameters, would be expected to have little effect. There is
considerable scope to improve the predictive performance
of thromboprophylaxis risk scores for use in CKD.
713
Recommendation 3.16.1: We recommend use of
non–vitamin K antagonist oral anticoagulants
(NOACs) in preference to vitamin K antagonists (e.g.,
warfarin) for thromboprophylaxis in atrial fibrilla-
tion in people with CKD G1–G4 (1C).
This recommendation puts high value on the use of NOACs, also
referred to as direct-acting oral anticoagulants or DOACs, in
people with CKD due to their simpler pharmacokinetic profile,
dosing, and monitoring than v itamin K antagonists and due to
their improved efficacy and relatively similar safety profile.
Although people with CKD G4 – G5 have been understudied in
RCTs, implementation in such groups can be achieved after
considering choice of NOAC and dosing.
Key information
Balance of benefits and harms. Benefits.
Data from 42,411
participants who received NOACs and 29,272 par ticipants
who received warfarin in 4 phase III tr ials were meta-analyzed
in 2014. Such trials largely excluded people with CKD G4–G5
but did include large numbers of participants with earlier
stages of CKD. Overall, NOACs significantly reduced the risk
of stroke or systemic embolic events by 19% compared with
warfarin (RR: 0.81; 95% CI: 0.73–0.91). This benefit was a
result largely from reduced r isk of hemorrhagic strokes (RR:
0.49; 95% CI: 0.38–0.64). There were large amounts of data
on stroke in those with a CrCl of <50 ml/min, and the
relative benefits were consistent and clearly evident in people
with CKD. There were also consistent effects in subgroup
analyses by age, sex, prior diabetes, prior stroke, and CHADS
2
score.
714
A more recent meta-analysis published in 2021 only
focused on subgroups with CKD and included data from 7
trials of NOACs versus warfarin in atrial fibrillation. It also
reported a 19% reduced risk of stroke/thromboembolic
complications in the NOAC group (HR: 0.81; 95% CI:
0.69–0.97).
715
Data in CKD G5 on dialysis were limited to
observational studies.
715
Our evidence review aimed to
collect information on subt ypes of outcome from subgroup
analyses repor ting results specifically in people with CKD.
Evidence of efficacy in the large trials is mainly for the
outcomes of stroke and hemorrhagic stroke, but our review
only found data from 3 trials for these outcomes resulting
in imprecise estimates of effect. The findings were
qualitatively consistent with the totality of the evidence
(Figure 41, Supplementa ry Table S14
716–721
).
Step 1
Diagnosis
• In people with CKD, use opportunistic pulse-based screening (e.g., taking at when measuring BP), followed by a
wearable device or Holter ECG testing
Step 2
Prophylaxis against
stroke and systemic
thromboembolism
(they are likely to have an increased CHA
2
DS
2
-VASc risk factor for stroke and are at high risk even with a score of 0–1)
managed (e.g., alcohol advice, use of a proton pump inhibitor)
Step 3
Rate/rhythm control
†
• Use medical therapy (e.g., beta blockade) to control ventricular rate to less than about 90 bpm at rest to decrease
symptoms and related complications
• For people with persistent symptoms despite adequate rate control, consider rhythm control with cardioversion,
antiarrhythmic therapy and/or catheter ablation
Figure 40 | Strategies for the diagnosis and management of atrial fibrillation. *Consider dose adjustments necessary in people with
chronic kidney disease (CKD).
†
The following has been recommended as a standard package for diagnostic evaluation of new atrial fibrillation:
(i) a 12-lead electrocardiogram (ECG) to establish the diagnosis, assess ventricular rate, and check for the presence of conduction defects,
ischemia, or structural heart disease; (ii) laboratory testing for thyroid and kidney function, serum electrolytes, and full blood count; and (iii)
transthoracic echocardiography to assess left ventricular size and function, left atrial size, for valvular disease, and right heart size and function.
BP, blood pressure; CHA
2
DS
2
-VASc, Congestive heart failure, Hypertension, Age $75 (doubled), Diabetes, Stroke (doubled), Vascular disease,
Age 65 to 74, and Sex category (female); HAS-BLED, Hypertension, Abnormal liver/kidney function, Stroke history, Bleeding history or
predisposition, Labile international normalized ratio (INR), Elderly, Drug/alcohol usage.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S241

Harms. The 2014 meta-analysis of 4 large phase III trials
found that NOACs reduced the risk of death from any
cause by 10%, confirming net safety (RR: 0.90; 95% CI:
0.85–0.95). Compared with warfar in, NOACs reduced the
risk of intracranial hemorrhage (defined as hemorrhagic
stroke, epidural, subdural, and subara chnoid hemorrhage)
by about one-half (RR: 0.48; 95% CI: 0.39–0.59), and the
risk of gastrointestinal bleeding was increased by about
one-quarter (RR: 1.25; 95% CI: 1.01–1.55). Overall, there
was no clear effect on the combination of these 2 safety
outcomes referred to as major bleeding (RR: 0.86; 95% CI:
0.73–1.00).
714
There were large amounts of data on major
bleeding in those with a CrCl of <50 ml/min, so
reassuring safety data clearly extended to people with
CKD. The re were also consistent safety data in subgroup
analyses by age, sex, prior diabetes, prior stroke, and
CHADS
2
score. There was a suggestion that major bleeding
was significantly reduced in people attending centers where
time in therapeutic international normalized ratio (INR) range
was <66% compared with centers with $66% time in range
(interaction P ¼ 0.02). This suggests that benefits of NOACs
are in part a result of their simpler pharmacokinetic profile
and dosing.
714
The 2021 meta-analysis that focused on CKD
subgroups from 7 trials found that bleeding events were also
not significantly different among those allocated NOACs
versus warfarin (HR: 0.83; 95% CI: 0.58–1.18).
715
Data in
CKD G5 on dialysis were limited to observational studies.
715
Our evidence review was again limited to a small number of
studies reporting subtypes of bleeding outcomes, and so
analyses found imprecise estimates of treatment effect. The
findings were qualitatively consistent with the totality of the
evidence (Figure 42, Supplementary Table S15
716–722
). The
review raised a hypothesis that some NOACs may be more
likely to reduce the risk of bleeding. However, given the
evidence of effect modification by time in therapeutic range in
the warfarin group, we have not provided specific
recommendations to prefer certain NOACs.
Certainty of evidence. The overall certaint y of the evidence
comparing NOACs with warfarin among people with CKD
and atrial fibrillation is low (Supplementary Tables S14
716–721
and S15
716–722
). Most of the RCTs evaluating the critical
outcomes were considered to have a low risk of bias. The
critical outcome of stroke was reported as any stroke,
ischemic stroke, and/or hemorrhagic stroke. Because there
Kidney
function Country
Follow-up
length Intervention* Control HR (95% CI)
eGFR 25–50
eGFR <50
CrCl 30–50
CrCl 30–49
CrCl 30–49
39 countries
44 countries
46 countries
Japan
45 countries
1.8 yr
1.8 yr
2.8 yr
2.5 yr
707 d
Apixaban 2.5–5 mg
Dabigatran 150 mg
Edoxaban 60 mg
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
Warfarin
Warfarin
Warfarin
0.86 (0.54, 1.35)
0.50 (0.28, 0.87)
0.99 (0.70, 1.40)
0.74 (0.17, 3.31)
1.02 (0.71, 1.46)
0.87 (0.69, 1.10)
NOTE: Weights are from random eects analysis
CrCl 30–50
CrCl 30–49
CrCl 30–49
46 countries
Japan
45 countries
2.8 yr
2.5 yr
707 d
Edoxaban 60 mg
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
Warfarin
0.91 (0.67, 1.24)
0.99 (0.29, 3.42)
0.95 (0.64, 1.41)
0.93 (0.73, 1.18)
CrCl 30–50
CrCl 30–49
CrCl 30–49
46 countries
Japan
45 countries
2.8 yr
2.5 yr
707 d
Edoxaban 60 mg
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
Warfarin
0.58 (0.30, 1.12)
1.98 (0.18, 21.80)
0.58 (0.23, 1.47)
0.62 (0.36, 1.04)
Author, year
Any stroke
†
Bohula, 2016
Hori, 2013
Fox, 2011
Subtotal (I
2
=0.0%, P=0.980)
Ischemic stroke
Stanifer, 2020
Hijazi, 2018
Bohula, 2016
Hori, 2013
Fox, 2011
Subtotal (I
2
=19.5%, P=0.291)
Hemorrhagic stroke
Bohula, 2016
Hori, 2013
Fox, 2011
Subtotal (I
2
=0.0%, P=0.619)
Weighted hazard ratio of stroke
0.2 0.5 512
Favors NOAC Favors control
Figure 41 | Pooled hazard ratio (HR) comparing non–vitamin K antagonist oral anticoagulants (NOACs) with warfarin among people
with chronic kidney disease in terms of stroke. Bohula E, Giugliano R, Ruff C, et al. Impact of renal function on outcomes with edoxaban in
the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36
716
; Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism
with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J.
2011;32:2387–2394
718
; Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in patients with atrial
fibrillation in relation to renal function over time—a RE-LY trial analysis. Am Heart J. 2018;198:169–177
719
; Hijazi Z, Alexander JH, Li Z, et al.
Apixaban or vitamin K antagonists and aspirin or placebo according to kidney function in patients with atrial fibrillation after acute coronary
syndrome or percutaneous coronary intervention: insights from the AUGUSTUS trial. Circulation. 2021;143:1215–1223
722
; Hori M, Matsumoto M,
Tanahashi N, et al. Safety and effi cacy of adjusted dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation: subanalysis of J-
ROCKET AF for patients with moderate renal impairment. Circ J. 2013;77:632–638
720
; Stanifer J, Pokorney S, Chertow G, et al. Apixaban versus
warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141:1384–1392.
721
CI, confidence interval; CrCl,
creatinine clearance; eGFR, estimated glomerular filtration rate.
chapter 3 www.kidney-international.org
S242
Kidney International (2024) 105 (Suppl 4S), S117–S314

were few stroke events reported across the RCTs, the certainty
of the evidence was downgrade d for imprecision.
Values and preferences. High value on the use of NOACs
included the conclusion that the simple dosing and lack of
INR monitoring compared with vitamin K antagonists would
lead to a substantial reduction in burden for those with an
indication for anticoagulation and their health services. There
is also good evidence for improved ef ficacy and a relatively
similar safety profile. Most fully informed people with CKD
would be expected to select a NOAC over a vitamin K
antagonist.
Resource use and costs. NOACs have been shown to be
cost-effective for stroke prevention in atrial fibrillation and
may even be cost-saving in people with CKD. Vitamin K
antagonist use may be associated with higher costs and
achieve fewer quality-adjusted life-years compared with
NOACs.
723
Considerations for implementation. A decision not to anti-
coagulate for thromboembolic prophylaxis due to low risk
would ideally be re-evaluated at each consultation and at least
every 6 months. When using antithrombotic therapy in
people with CKD, it is prudent to treat modifiable risk factors
for bleeding (e.g., alcohol intake) and use gastroprophylaxis
with a PPI, particularly when combined with antiplatelet
therapy.
Rationale
A number of large RCTs demonstrated that NOACs reduce
the risk of intracranial bleeding compared with warfarin and,
overall, modestly reduce mortalit y in people with atrial
Kidney
function Country
Follow-up
length Intervention* Control HR (95% CI)
CrCl 30–50
eGFR 25–50
eGFR <50
CrCl 30–50
CrCl 30–49
CrCl 30–49
33 countries
39 countries
44 countries
46 countries
Japan
45 countries
6 mos
1.8 yrs
1.8 yrs
2.8 yrs
2.5 yrs
707 dys
Apixaban 2.5–5 mg
Apixaban 2.5–5 mg
Dabigatran 150 mg
Edoxaban 60 mg
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
Warfarin
Warfarin
Warfarin
Warfarin
0.51 (0.28, 0.93)
0.59 (0.45, 0.77)
1.11 (0.87, 1.14)
0.76 (0.58, 0.98)
0.89 (0.36, 2.18)
0.98 (0.73, 1.30)
0.80 (0.61, 1.05)
NOTE: Weights are from random eects analysis
CrCl 30–50
eGFR 25–50
CrCl 30–49
CrCl 30–49
33 countries
39 countries
Japan
45 countries
6 mos
1.8 yrs
2.5 yrs
707 dys
Apixaban 2.5–5 mg
Apixaban 2.5–5 mg
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
Warfarin
Warfarin
0.59 (0.41, 0.84)
0.35 (0.17, 0.72)
1.22 (0.78, 1.91)
0.98 (0.85, 1.15)
0.76 (0.51, 1.14)
CrCl 30–50
CrCl 30–49
46 countries
Japan
2.8 yrs
2.5 yrs
Edoxaban 60 mg
Rivaroxaban 10 mg
Warfarin
Warfarin
0.48 (0.22, 1.07)
1.04 (0.07, 16.70)
0.51 (0.24, 1.09)
CrCl 30–50
CrCl 30–49
46 countries
45 countries
2.8 yrs
707 dys
Edoxaban 60 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
0.46 (0.26, 0.82)
0.82 (0.41, 1.60)
0.60 (0.34, 1.05)
Author, year
All clinically relevant bleeding
†
Hijazi, 2021
Stanifer, 2020
Hori, 2013
Fox, 2011
Subtotal (I
2
= 79.9%, P=0.002)
Fatal bleeding
Bohula, 2016
Hori, 2013
Subtotal (I
2
= 0.0%, P=0.595)
Major bleeding
†
Hijazi, 2021
Stanifer, 2020
Hijazi, 2018
Bohula, 2016
Hori, 2013
Fox, 2011
Subtotal (I
2
= 79.0%, P=0.000)
Intracranial hemorrhage
Bohula, 2016
Fox, 2011
Subtotal (I
2
= 38.2%, P=0.203)
Hori, 2013
Fox, 2011
Subtotal (I
2
= 14.1%, P=0.281)
CrCl 30–49
CrCl 30–49
Japan
45 countries
2.5 yrs
707 dys
Rivaroxaban 10 mg
Rivaroxaban 20 mg
Warfarin
Warfarin
1.35 (0.82, 2.22)
1.01 (0.85, 1.20)
1.06 (0.86, 1.31)
Clinically relevant nonmajor bleeding
‡
Weighted hazard ratio of bleeding
0.2 0.5 512
Favors NOAC Favors control
Figure 42 | Pooled hazard ratio (HR) comparing non–vitamin K antagonist oral anticoagulants (NOACs) with warfarin among people
with chronic kidney disease in terms of bleeding. Bohula E, Giugliano R, Ruff C, et al. Impact of renal function on outcomes with edoxaban in
the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36
716
; Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic embolism
with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J.
2011;32:2387–2394
718
; Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran compared with warfarin in patients with atrial
fibrillation in relation to renal function over time—a RE-LY trial analysis. Am Heart J. 2018;198:169–177
719
; Hori M, Matsumoto M, Tanahashi N,
et al. Safety and efficacy of adjusted dose of rivaroxaban in Japanese patients with non-valvular atrial fibrillation: subanalysis of J-ROCKET AF for
patients with moderate renal impairment. Circ J. 2013;77:632 –638
720
; Stanifer J, Pokorney S, Chertow G, et al. Apixaban versus warfarin in
patients with atrial fibrillation and advanced chronic kidney disease. Circulation. 2020;141:1384–1392
721
; Hijazi Z, Alexander JH, Li Z, et al.
Apixaban or vitamin K antagonists and aspirin or placebo according to kidney function in patients with atrial fibrillation after acute coronary
syndrome or percutaneous coronary intervention insights from the AUGUSTUS trial. Circulation. 2021;143:1215–1223.
722
CI, confidence interval;
CrCl, creatinine clearance; eGFR, estimated glomerular filtration rate.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S243

fibrillation. The y offer benefits in terms of ease of monitoring.
CKD does not appear to importantly modify these benefits, at
least down to G4.
Practice Point 3.16.2: NOAC dose adjustment for GFR is
required, with caution needed at CKD G4–G5.
Doses of NOACs may need to be modified in people with
decreased GFR taking into consideration the age, weight, and
GFR of a person with CKD (Figure 43
710
). Consult the
relevant summaries of product characteristics for the latest
information on dosing (Chapter 4).
Practice Point 3.16.3: Duration of NOAC discontinuation
before elec tive procedures needs to consider procedural
bleeding risk, NOAC presc ribed, and level of GFR
(Figure 44).
710,724
eCrCl (ml/min)
a
Warfarin Apixaban
b
Dabigatran Edoxaban
c
Rivaroxaban
>95 Adjusted dose (INR 2–3) 5 mg b.i.d. 150 mg b.i.d. 60 mg QD
d
20 mg QD
51–95 Adjusted dose (INR 2–3) 5 mg b.i.d. 150 mg b.i.d. 60 mg QD 20 mg QD
31–50 Adjusted dose (INR 2–3) 5 mg b.i.d.
150 mg b.i.d. or
110 mg b.i.d.
e
30 mg QD 15 mg QD
a
eCrCl (ml/min)
a
Warfarin Apixaban
b
Dabigatran Edoxaban Rivaroxaban
15–30 Adjusted dose for INR
2–3 could be considered
2.5 mg PO b.i.d.
could be considered
Unknown
(75 mg PO b.i.d.)
f,g
30 mg QD
h
could be considered
15 mg QD
could be considered
<15 not on dialysis Equipoise based on
observational data
and meta-analysis
Unknown
(2.5 mg PO b.i.d.)
f
Not recommended Not recommended Unknown
(15 mg QD)
f
<15 on dialysis Equipoise based on
observational data
and meta-analysis
Unknown
(2.5 mg PO b.i.d.)
f
Not recommended Not recommended Unknown
(15 mg QD)
f
b
Figure 43 | Evidence from (a) randomized controlled trials (RCTs) regarding therapeutic anticoagulation dose by glomerular filtration
rate (GFR) and (b) in areas where RCTs are lacking. Dosing of non–vitamin K antagonist oral antic oagulants (NOACs) based solely on
limited pharmacokineti c and pharmacodynam ic data (i.e., there are no randomized efficacy or safety trial data assessing clinical outcomes
for stroke thromboprophylaxis in atrial fibrillation at chronic kidney disease [CKD] G4–G5).
a
Cockcroft-Gault estimated creatinine clearance
(eCrCl).
b
Apixaban dose modification from 5 mg twice per day (b.i.d.) to 2.5 mg b.i.d. if a person has any 2 of the following: serum
creatinine $1.5 mg/dl (133
m
mol/l), age $80 years, or body weight #60 kg.
c
In the Effective Anticoagulation With Factor Xa Next Generation
in Atrial Fibrillation–Thrombolysis in Myocard ial Infarction 48 (ENGAG E-A F TIMI 48) study, the dose was halved if any of the following: eCrCl
of 30– 50 ml/min, body weight #60 kg, or concomitant use of verapamil or quinidine (potent P-glycoprotein inhibitors).
d
This dose has not
been a pproved f or use by the US Food and Drug Admin istration (FDA) in this category of GFR.
e
In countries where 110 mg b.i.d. is approved,
healthcare providers may prefer this dose after clinical assessment of thromboembolic versus bleeding risk. This dose has not been
approved for use by the US FDA.
f
NOAC doses listed in parenthesis are doses that do not currently have any clinical or efficacy data. The
doses of NOACs apixaban 5 mg b.i.d.,
b
rivaroxaban 15 mg every day, and dabigatran 75 mg b.i.d. are included in the US FDA–approved
labeling based on limited dose pharmacokinetic and pharmacodyn ami cs data with no clinical safety data. We suggest consideration of the
lower dose of apixaban 2.5 mg oral b.i.d. in CKD G5 and G5D to r educe bleeding risk until clinic al safety data are avail able.
g
Dabigatran 75
mg available only in the United States.
h
The dose was halved if any of the following: estimated CrCl of 30–50 ml/min, body weight of #60 kg,
orconcomitantuseofverapamilorquinidine(potentP-glycoprotein inhibitors). INR, international normalized ratio; QD, every day.
Reproduced from Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease:
Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;39:2314–2325.
710
ª The Author(s) 2018. Published by
Oxford University Press on behalf of the European Society of Cardiology. This is an Open Access article distributed under the terms of the
Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0 /).
chapter 3 www.kidney-international.org
S244
Kidney International (2024) 105 (Suppl 4S), S117–S314

Apixaban–Edoxaban–RivaroxabanDabigatran
Low risk High risk
No important bleeding risk and/or adequate local hemostasis possible:
perform at trough level (i.e., ≥12 or 24 h after last intake)
Low risk High risk
CrCl ≥80 ml/min ≥24 h ≥48 h ≥24 h ≥48 h
CrCl 50–80 ml/min ≥36 h ≥72 h ≥24 h ≥48 h
CrCl 30–50 ml/min
a
≥48 h ≥96 h ≥24 h ≥48 h
CrCl 15–30 ml/min
a
No ocial indication No ocial indication ≥36 h ≥48 h
CrCl <15 ml/min No ocial indication for use
There is no need for bridging with LMWH/UFH
Figure 44 | Advice on when to discontinue non–vitamin K antagonist oral anticoagulants (NOACs) before procedures (low vs. high risk).
The bold values deviate from the common stopping rule of $24-hour low risk, $48-hour high risk. Low risk is defined as a low frequency of
bleeding and/or minor impact of a bleed. High risk is defined as a high frequency of bleeding and/or important clinical impact. Adapted from
Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart Rhythm Association practical guide on the use of non–vitamin-K
antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2017;38:2137–2149.
724 a
Many of these
people may be on lower dose of dabigatran (110 mg twice per day [b.i.d.]) or apixaban (2.5 mg b.i.d.), or have to be on the lower dose of
rivaroxaban (15 mg QD) or edoxaban (30 mg QD). Dabigatran 110 mg b.i.d. has not been approved for use by the US Food and Drug
Administration. CrCl, creatinine clearance, LMWH, low-molecular-weight heparin; UFH, unfractionated heparin. Reproduced from Turakhia MP,
Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes
(KDIGO) Controversies Conference. Eur Heart J. 2018;39:2314–2325.
710
ª The Author(s) 2018. Published by Oxford University Press on behalf of
the European Society of Cardiology. This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-
Commercial License (http://creativecommons.org/licenses/by-nc/4.0/).
For research recommendations, please see Chapter 6: Research recommendations.
www.kidney-international.org chapter 3
Kidney International (2024) 105 (Suppl 4S), S117–S314 S245

Chapter 4: Medication management and drug
stewardship in CKD
Medication management is an important component of the
care of people with CKD. Medications can be highly benefi-
cial, but some may be toxic, are excreted by the kidney, may
have narrow therapeutic w indows, or may have no proven
clear evidence of benefit or indication in people with CKD.
Drug stewardship refers to the effective, safe, and sus-
tainable use of medications by all staff and physicians,
encompassing the whole cycle of medication u se. Medica-
tions need to be prescribed responsibly, monitored for effi-
cacy and safet y, and when they do not or no longer ser ve
their intended purpose, discontinued. This chapter discusses
key concepts in the processes o f dr ug stewardship in people
with CKD. It is beyond the scope of this guideline to list all
the medications that may have altered risks/benefits in
people with CKD. Such information is widely available in
documents that may exist at local, regional, or national
bodies (e.g ., British National Formulary : www.bnf.org)and
in textbooks of pharmacology. However, we describe case
examples to h ighlight the key classes of commonly pre-
scribed medic ations in peop le wi th CKD. This guidance is
based on knowledge of pharmacology that has universal
relevance. In many cases, knowledge of altered risks/benefits
of medications comes, however, from observational studies
and case reports from routine care.
4.1 Medication choices and monitoring for safety
Abnormal kidney function results in alteration in pharma-
cokinetics and pharmacodynamics, and for people with CKD,
as the GFR worsens, so does the prevalence of polypharmacy
and comorbidities.
725
People with CKD are at increased risk
of medication errors and inappropriate prescribing (noted
to be up to 37% in ambu latory outpatient studies and up
to 43% in long-ter m care studies
726,727
). Thus, improved
understanding and collaboration with pharmacists in
developing care plans and medication review is strongly
recommended.
People with CKD have reduced ability to excrete medica-
tions and/or their metabolites (which may increase adverse
event risk or exaggerate/diminish efficacy) and increased
sensitivity to medications (e.g., those bound to albumin in
hypoalbuminemic states such as nephrotic syndrome).
Additional issues include nephrotoxicity, diminished toler-
ance of side effects in the context of coexisting comorbidities
or older age, and lack of adequate evidence for either benefit
or harm of specific compounds, due to historical exclusion of
people with (advanced) CKD from most clinical trials.
727,728
As in all medical decision-making, healthcare providers
should consider the indication, benefit-risk profile, and po-
tential nephrotoxicity while balancing accessibility, availabil-
ity, local health policies, cultural practices, affordability, and
patient preferences. Where available, consultation with
pharmacists as part of the multidisciplinary team is encour-
aged to assure optimized comprehensive medication man-
agement and to improve pharmacoequity.
Practice Point 4.1.1: People with CKD may be more sus-
ceptible to the nephrotoxic effects of medications. When
prescribing such medications to people with CKD, always
consider the benefits versus potential harms.
Between 18%–20% of people with CKD G3 – G5 receive at
least one potentially inappropriate nephrotoxic medication
annually, primarily NSAIDs, nephrotoxic antivirals, and
bisphosphonates.
729
Nephrotoxic medications may be
indicated in people with CKD if expected benefits exceed
potential harms.
730
However, whenever possible, healthcare
providers should strive to use non-nephrotoxic alternatives.
Common nephrotoxic medications to be aware of and
potential alternatives that could be prescribed instead are
listed in Table 31.
725,731–738
Although some nephrotoxic
medications have viable alternatives, the alterna tives may be
less potent or there is limited comparison data on clinical
outcomes, safety, and cost-effectiveness.
Practice Point 4.1.2: Monitor eGFR, electrolytes, and ther-
apeutic medication levels, when indicated, in people with
CKD receiving medications with narrow therapeutic win-
dows, potential adverse effects, or nephrotoxicity, both in
outpatient practice and in hospital settings.
Ensuring a safe use of medication requires careful moni-
toring for adverse effects and efficacy. A key example includes
the need to monitor potassium and creatinine during the
initial weeks of treatment with ACEi and ARBs (Figure 21 ).
23
Medications such as gentamicin and vancomycin have a
narrow therapeutic range, with higher trough levels
commonly associated with AKI, and so require close
monitoring of GFR and medication levels during prolonged
treatment.
731
Other medications, such as lithium or
methotrexate, require at least annual monitoring of
creatinine to evaluate potential risks of nephrotoxicity.
Practice Point 4.1.3: Review and limit the use of over-the-
counter medicines and dietary or herbal remedies that may
be harmful for people with CKD.
chapter 4 www.kidney-international.org
S246
Kidney International (2024) 105 (Suppl 4S), S117–S314

Kidney disease can be induced or accelerated by the use of
certain over-the-counter (OTC) medications, herbal rem-
edies, and other dietar y supplements. One of the most used
class of OTC analgesic medications is NSAIDs. NSAIDs are
associated with interstitia l nephritis, analgesic nephropathy,
and hypertension.
739
Indiscriminate chronic OTC NSAID use
has been associated with a higher risks of kidney failure
compared with nonuse and should be discouraged.
740–743
However, judicious NSAID use, under careful supervision of
a nephrologist, may be preferred to other pain medications
such as opioids that have stronger associations with adverse
events.
744,745
PPIs are also common OTC medications in
some countries that have been associated with AKI and
CKD due to tubulointerstitial nephritis and acute interstitial
nephritis.
733,734
The use of herbal compounds remains highly prevalent in
some countries and cultures.
746
These products are often used
in an unmonitored setting without the inpu t of healthcare
providers. Many of the se remedies are composed of natural
compounds with complex active ingredients that have not
been evaluated in people with CKD and/or that may lead to
many different adverse effects. The frequency of CKD
associated with herbal remedy use is not known and is
likely different in different parts of the world, depending on
local availability and reasons for use. Examples include
aristolochic acid nephropathy or nephrotoxicity due to
alkaloid compounds often found in Chinese herbal
remedies.
747
However, cases of nephrotoxicity have been
reported for many other herbal remedies globally.
746,748,749
The potential toxicity of herbal remedies may be enhanced
by coexisting volume depletion and by other illness or
medication use.
Dietary supplements are readily available in most countries
around the world and are usually not classified as OTC med-
ications. Because of this, their regulation for identity and safety
can vary widely. Although laws pertaining to dietary supple-
ment labeling prohibit specific claims for the treatment or
prevention of disease, these products are widely used as
“alternative” or “complementary” therapy. Patients and pro-
viders often assume that these products are at least safe and
possibly effective. Their pharmacokinetics may be unknown
and potential toxicity unstudied. Classic examples include
creatine supplements used for body building that have been
associated with allergic interstitial nephritis.
750,751
Another
example is vitamin C (ascorbic acid) supplements, which in
excess can lead to tubular calcium oxalate crystal deposition.
752
Healthcare providers are encouraged to routinely inquire
about the use of herbal remedies and recommend stopping
any unprescribed alternative remedy that may pose a threat
for (kidney) health. Figure 45
747,753,754
lists common herbal
remedies and dietary supplements arranged by the countries
where the adverse effects were reported to increase
awareness and facilitate discussions.
Special considerations
Global access to medications.
Access to medications varies
globally. Approximately 30% of the world population lacks
timely access to quality medications. The International Soci-
ety of Nephrology (ISN) reports that only 35% of patients in
low-resource settings have access to ACEi/ARBs, statins, and
Table 31 | Key examples of common medications with documented nephrotoxicity and, where available, selected non-
nephrotoxic alternatives
Nephrotoxic medication Potential non-nephrotoxic alternatives
Analgesics
NSAIDs: nephrotoxic effects include a decrease in GFR through a reduction in
prostaglandin-dependent kidney blood flow, allergic interstitial nephritis (AIN),
and nephrotic syndrome
725
Acetaminophen
Antimicrobials
Aminoglycosides: accumulates in the proximal tubular cells and disrupts phospholipid
metabolism, resulting in cell apoptosis and acute tubular necrosis (ATN)
731,732
Cephalosporins and carbapenems
Vancomycin: unclear cause of nephrotoxicity, but likely related to ATN and possible AIN
731,732
Linezolid and daptomycin
731
Sulfamethoxazole-trimethoprim: AIN, ATN, crystalluria within the distal convoluted
tubule and reversible inhibition of tubular creatinine secretion
731
Clindamycin þ primaquine, pentamidine,
and atovaquone
Gastrointestinal medications
Proton pump inhibitors: may result in AKI and CKD due to tubulointerstitial nephritis
and AIN
733,734
H2-receptor antagonists
Cardiovascular medications
Warfarin: glomerular hemorrhage, oxidative stress causing kidney tubular damage, and
direct effects on kidney vascular calcification by vitamin K–dependent alterations
of matrix Gla protein
735,736
Non–vitamin K antagonist oral anticoagulants
Other
Lithium: nephrogenic diabetes insipidus as well as CKD from chronic tubulointerstitial
nephropathy
737
Aripiprazole, lamotrigine, quetiapine, valproate
CKD, chronic kidney disease; GFR, glomerular filtration rate; NSAID, nonsteroidal anti-inflammatory drug.
www.kidney-international.org chapter 4
Kidney International (2024) 105 (Suppl 4S), S117–S314 S247

insulin.
755
There are also numerous barriers to additional
important medications for the management of CKD
complications, such as erythropoietin analogs, iron infusion,
and phosphate or potassium binders.
There are growing concerns regarding the use of falsified
and substandard medications in low- to lower-middle–in-
come countries as the y pose potential harm, particularly to
those people at risk of and with CKD. Patients and their
families should be aware that medication falsification is often
associated with illicit internet supply. Many vulnerable com-
munities and people with low health literacy and those in
countries with less rigorous regulatory systems are more at
risk of medication falsification. Therefore, increased global
awareness is important, and people with CKD should be
provided with appropriate education and follow-up with
relevant support in accordance with local health policies.
Medications and pregnancy.
Practice Point 4.1.4: When prescribing medications to
people with CKD who are of child-bearing potential, always
review teratogenicity potential and provide regular repro-
ductive and contraceptive counseling in accordance with
the values and preferences of the person with CKD.
When pregnancy is not desired, we note that while the
effect of different forms of contraception on GFR is un-
known,
756
oral contraceptives are associated with increased
BP and hypertension.
757
Nonoral hormonal contraceptives
have a less clear impact on BP.
757
Pregnancy may pose a risk of CKD progression for people
with established CKD. In addition, some recommended medi-
cations to slow or prevent CKD progression are teratogenic (such
as ACEi/ARBs or mammalian target of rapamycin inhibitors)
and discontinuation during pregnancy should be considered.
758
Some CKD-specific medications should be continued during
pregnancies such as hydroxychloroquine, tacrolimus,
cyclosporin, eculizumab, prednisone, azathioprine, colchicine,
and intravenous immunoglobulin. A thorough medication
chart review is necessary to replace teratogenic medications
before conception, or whenever this is not possible, ensure a
strict monitoring plan with cessation of potentially teratogenic
medications at conception.
759
A similar approach should be
undertaken during lactation recognizing that some
medications suitable for use during pregnancy may not be
appropriate for lactation, and vice versa.
760
Multidisciplinary
care with obstetrics and potentially other subspecialty care is
required before conception and throughout pregnancy and
lactation.
54
Sex-specific aspects of medication use in CKD. Sex differences
in medication safety and efficacy in people with CKD are
understudied,
38,761,762
For example, sex differences in body
weight and composition as well as physiological functions
Tripterygium
Chimonanthus
Tetrandra
Menispermi
Strychnos
Wood veratry
Aconitum
Groundsel
Monkhood
Bee pollen
Fish gallbladder
Cyprinidae (grass carp, common carp, silver carp,
black shark sh, bony-lipped barb sh)
Indian carp (Labeo rohita)
Mourning cypress (Cupressus funebris)
Snake gallbladder (Naja naja atra)
Star fruit (Averrhoa carambola)
Oduvan (Cleistanthus collinus)
Yellow oleander (Thevetia peruviana)
Djenkol beans, jering (Pithecolobium lobatum)
Cone ower (Echinacea)
Spurge (Euphorbia matabelensis)
Khat leaf (Catha edulis)
Cape aloe (Aloe capensis)
Impila, ox-eye daisy (Callilepis laureola)
Potassium dichromate
Wild wisteria, violet tree
(Securidaca longipedunculata)
Paraphenylene diamine (PPD)
Takaout roumia
Sheep bile
Bird ower (Crotalaria laburnifolia)
Worldwide:
Licorice (Glycyrrhiza glabra)
Mushrooms (Amanita phalloides, Cortinarius spp.)
Aristocholic acid-containing Chinese herbs
Alfafa (Medicago sativa L.)
Black cohosh (Actaea racemosa)
Cone ower (Echinacea)
Chromium picolinate
CKLS (colon, kidney, liver, spleen
purier contains Aloe vera, Cascara
sagrada, Larrea tridentata and
Arctostaphylos uva-ursi)
Creatine
Hemlock (Conium maculatum)
Ma huang (Ephedra sinica)
Hydrazine sulfate
Noni juice (Morinda citrifolia)
St. John’s wort (Hypericum perforatum)
Wormwood oil (Artemisia absinthium)
L-lysine
Chaparral (Larrea tridentata)
Propolis
Star fruit (Averrhoa carambola)
Cat’s claw (Uncaria tomentosa)
Hemlock (Conium maculatum)
Noni juice (Morinda citrifolia)
Senna fruit tea (Sennae fructus angustifoliae)
Germanium
Hydrazine sulfate
Willow bark (Salix daphnoides)
Anatolian hawthorn (Crataegus orientalis)
Tribulus terrestris
Figure 45 | Selected herbal remedies and dietary supplements with evidence of potential nephrotoxicity, grouped by the continent
from where the reports first came. Data from Yang B, Xie Y, Guo M, et al. Nephrotoxicity and Chinese herbal medicine. Clin J Am Soc Nephrol.
2018;13:1605–1611
747
; Gabardi S, Munz K, Ulbricht C. A review of dietary supplement-induced renal dysfunction. Clin J Am Soc Nephrol.
2007;2:757–765
753
; Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin J Am Soc Nephrol. 2018;13:1897–1908.
754
chapter 4 www.kidney-international.org
S248
Kidney International (2024) 105 (Suppl 4S), S117–S314

may impact drug metabolism and response. Because drug
dosages are often universal, women are more likely to
consume higher doses in relation to their body
weight,
136,763,764
and this could be associated with more
adverse events.
136
In people with heart failure with reduced
ejection fraction, observational studies show improved
sur vival in women with lower doses of renin-angiotensin-
aldosterone system (RAAS)-blocking medications, whereas
men bene fit from higher doses.
765,766
This may be related to
lower RAAS activit y in women compared with men.
767
4.2 Dose adjustments by level of GFR
Practice Point 4.2.1: Consider GFR when dosing medications
cleared by the kidneys.
Many medications and/or their active metabolites are
excreted by the kidneys. Failure to properly account for the
effect of GFR when designing appropriate drug-dosing regi-
mens can predispose a person to treatment failure or adverse
events.
725,728
Although guidelines for adjustment of the
dosing regimen at varying severities of CKD provided by
the manufacturer are widely available in pharmacopeias,
textbooks, online references, or local procedures, there may
be significant differences in information provided by these
resources.
768
Practice Point 4.2.2: For most people and clinical settings,
validated eGFR equations using SCr are appropriate for
drug dosing.
Practice Point 4.2.3: Where more accuracy is required for
drug-related decision-making (e.g., dosing due to narrow
therapeutic or toxic range), drug toxicity, or clinical situ-
ations where eGFR cr estimates may be unreliable, use of
equations that combine both creatinine and cystatin C, or
measured GFR may be indicated.
An assessment of GFR is important for guiding decisions
related to the choice and dosing of medications. Section 1.2
addresses the accuracy of validated eGFR equations, as well
as indications for the use of eGFRcr-cys or mGFR.
There is inconsistency between this guidance and those
found in the package inser ts or classic source references for
drug dosing. Regulatory agencies have not universally
required pharmacokinetics in abnormal kidney function for
medication approval.
769
In addition, although the Cockcroft-
Gault formula for estimating CrCl has been used in many past
pharmacokinetic studies that serve as the basis for the drug
dosing, there are multiple concerns with that equation. It
was developed in an era when the need for standardization
of creatinine measurements was not appreciated, women
and individuals of Black race were not included, and there
are concerns about use of weight, which can be impacted
by edema or obesity.
770
However, to date, few studies have
been conducted to compare different equations for eGFR in
the context of drug dosing/kinetics, etc.
There is now a recognition by major regulatory agencies
that “any contemporary, widely accepted, and clinically
applicable estimating GFR equation is considered reasonable
to assess GFR in pharmacokinetic studies.”
770,771
Practice Point 4.2.4: In people with extremes of body
weight, eGFR nonindexed for body surface area (BSA) may
be indicated, especially for medications with a narrow
therapeutic range or requiring a minimum concentration
to be effective.
For assessment of CKD, it is relevant to compare the GFR
according to a standard body size. For this reason, GFR
estimating equations have been developed in units of ml/min
per 1.73 m
2
. However, because drug clearance is more
strongly associated with nonindexed eGFR (ml/min) than
indexed eGFR (ml/min per 1.73 m
2
), in very smal l or large
individuals, this can result in over- or underdosing, respec-
tively, as well as noninitiation of certain medications.
772,773
Nonindexed eGFR can be obtained by multiplying the
indexed eGFR results by the person’s BSA and dividing by
1.73 m
2
, or by using an appropriate online calculator.
Practice Point 4.2.5: Consider and adapt drug dosing in
people where GFR, non-GFR determinants of the filtration
markers, or volume of distribution are not in a steady state.
In people with rapidly changing health status, it can be a
challenge to estimate the GFR. Serum concentrations of
filtration markers may be changing because of changes in true
GFR and/or in non-GFR determinants of the marker (Section
1.2). In such settings for people who require medications that
are impacted by or could impact GFR, healthcare providers
should regularly assess risk, benefits, and value of the
medication, and consider higher or lower doses than
indicated. Where possible, use medication level testing to
guide dosing.
730,774
Special considerations
Dose adjustments in cancer.
GFR plays a large role in
determining anticancer therapy, including anticancer agent
selection, dosing, and eligibility for investigational drugs and
clinical trials.
775,776
Notwithstanding its lack of validation and
its relative inaccuracy compared with other validated eGFR
equations, the Cockroft-Gault equation continues to be one
of the most commonly used eGFR methods for these
people.
775,776
An evaluation of eGFR equation performance
against mGFR determined by plasma clearance of
51
Cr-
EDTA in 1200 people with solid tumors observed the
eGFRcr (CKD-EPI) and the eGFRcr-cys (CKD-EPI)
predicted mGFR with greater accuracy than Cockroft-
Gault.
136
We advise that the same approach to GFR
evaluation described in Section 1.2 be adopted in oncology
practice and clinical trials.
136,764
BSA-adjusted eGFR may be
indicated for selected specific situations like carboplatin
dosing. It is important to consider that non-GFR
determinants of both creatinine and cystatin C may be
www.kidney-international.org chapter 4
Kidney International (2024) 105 (Suppl 4S), S117–S314 S249

more profound in people with cancer, and mGFR may be the
preferred method to guide the initial dosing for a select group
of anticancer drugs including, but not limited to, carboplatin,
cisplatin, and methotrexate (Section 1.2).
Dose adjustment in children/neonates. In addition to the
usual weight-based dosing for children, specific guidance on
drug dosing should be followed for neonates who have lower
GFR than those outside the neonatal period.
Dose adjustment in pregnancy. Creatinine decreases physi-
ologically during pregnancy due to glomerular hyper-
filtration, and BSA varies. This creates challenges for using
GFR or eGFR equations.
54
In such settings for people who
require medications that are impacted by or could impact
GFR, healthcare providers should regularly assess r isk,
benefit, and value of medications.
4.3 Polypharmacy and drug stewardship
People with CKD are particularly susceptible to poly-
pharmacy due to multiplicity of comorbidities and mu ltiple
physician or health system encounters related to those. Most
people with CKD not treated with dialysis receive 6–12
different medications per day.
763
Polypha rmacy leads to
increased pill burden and potential harm due to medication
errors and drug-drug interactions. Thus, healthcare
providers, including clinical pharmacists, should be diligent
in assessing medication appropriateness, number, dose, and
potential interactions. Drug stewardship promotes safe
medication use throughout the course of therapy.
Medications need to be prescribed responsibly, monitored
for efficacy and safety, and when no longer required,
discontinued.
Practice Point 4.3.1: Perform thorough medication review
periodically and at transitions of care to assess adherence,
continued indication, and potential drug interactions
because people with CKD often have complex medication
regimens and are seen by multiple specialists.
Medication review is essential for minimizing the occur-
rence of medication-related problems (e.g., inappropr iately
high doses and drug interactions) that commonly occur in the
CKD population.
777
If a person no longer has an indication
for a medication that may contribute to kidney injury (e.g.,
PPIs), healthcare providers should recognize the
opportunity to discontinue the medication. Medication
review at each clinical encounter, especially care transitions,
is an opportunity to review medication types, interval, and
doses especially if the individual has experienced a decline
in GFR (e.g., metformin) or physiologic changes that can
impact medication volume of distribution (e.g., volume
overload and sarcopenia).
778
Figure 46
729
discusses key steps
in the medication review process. Three studies have
Assessing that the dosage
and regimen are correct
Medication agreement
Communication with other physicians
CKD
patient
Optimizing
the
medication
impact
Minimizing
medication-
related
problem
Reviewing the medication list
for interactions or adverse eects
Ensuring that proper
monitoring takes place
Assessing medication adherence
and causes for nonadherence
Obtaining an accurate
medication list
Evaluating whether each medication
is necessary or whether any other
necessary medication is required
Determining whether each
medication is the preferred
medication for its indication
Resolving any discrepancies
between the actual medication list
and the one in the medical record
M
e
d
i
c
a
t
i
o
n
r
e
c
o
n
c
i
l
i
a
t
i
o
n
M
e
d
i
c
a
t
i
o
n
r
e
v
i
e
w
M
e
d
i
c
a
t
i
o
n
r
e
v
i
e
w
Figure 46 | Suggested steps in the process of medication review and reconciliation. Best practices for medication review and reconciliation
in people with chronic kidney disease (CKD) include 8 steps
728
and can be summarized as follows: (i) obtain an accurate medication list from the
patient; (ii) evaluate whether all medications are medically necessary or whether any other medications is required; (iii) assess whether current
therapy represents the “drug of choice” for each indication, individualized for each patient; (iv) evaluate the medication dosage and regimen,
taking into consideration related factors such as liver dysfunction, patient size, or weight (e.g., amputation, muscle wasting, and over- or
underweight); (v) review the medication list for drug interactions, including drug-drug, drug-disease, drug-laboratory, and drug-food
interactions; (vi) ensure that proper monitoring takes place; (vii) determine whether there are any barriers to patient adherence, and evaluate
relevant laboratory values; and (viii) identify and resolve any discrepancies between the medications list and the one in the medical record;
communication of performed changes in the medication chart with other physicians is necessary given the role of multiple prescribers involved
in the care of patients with CKD.
729
chapter 4 www.kidney-international.org
S250
Kidney International (2024) 105 (Suppl 4S), S117–S314

evaluated medication review by clinical practices in people
with CKD, observing reductions in the use of inappropriate
medications and medication-related problems, both in
outpatient and inpatient settings.
765,766
The most frequent
reviews involved altering dosage or dose interval and
discontinuing NSAIDs. More frequent medication reviews
may be needed in older adults with complex medication
regimens compared with younger people with CKD.
In the context of good drug stewardship, healthcare pro-
viders should be aware of the issue of “prescribing cascade.” A
prescribing cascade is a sequence of events that begins when
an adverse event is misinterpreted as a new medical condition
and a subsequ ent drug is prescribed to treat this adverse
event.
779
Before prescribing new medications to address
newly reported symptoms, it is important to first assess if
the symptoms represent a side effect from an existing
medication. An example of a prescribing casca de is as
follows: peripheral edema because of calcium channel
blocker may be managed by initiation of a new medication
(i.e., diuretic), which can lead to addition al adverse
reactions (e.g ., hypokalemia and dizziness).
Practice Point 4.3.2: If medications are discontinued during
an acute illness, communicate a clear plan of when to
restart the discontinued medications to the affected person
and healthcare providers, and ensure documentation in the
medical rec ord.
Sick day rules have been endorsed as useful guidance to people
with CKD in the setting of acute, dehydrating illness. Specifically,
patients receive guidance to temporarily stop the following
medications: sulfonylur eas, ACEi, diuretics/direct renin in-
hibitors,metformin,ARBs,NSAIDs,andSGLT2i(oftendescribed
with the acronym SADMANS).
780
However , there is a paucity of
evidence to support sick day rules to prevent AKI or other
clinically relevant outcomes.
781,782
Instead, data suggest
potential harm if people mak e mistakes in recognizing
dehy drating illness or about which drugs to stop and when to
restart.
783
Figure 47 shows the steps that must occur correctly
for sick day rules to be implemented appropriately. The most
reported problem is failure to restart the medication.
784
The
plan to restart medications should be detailed in the medical
recor ds and clearly communicated to the patients. Patients may
additionally benefitfrommedicationreviewwithinamonthto
ensure appropriate medications are restarted.
Practice Point 4.3.3: Consider planned discontinuation of
medications (such as metformin, ACEi, ARBs, and SGLT2i)
in the 48–72 hours prior to elective surgery or during the
acute management of adverse effects as a precautionary
measure to prevent complications. However, note that
failure to restart these medications after the event or pro-
cedure may lead to unintentional harm (see Practice Point
4.3.2).
The rationale for temporary discontinuation of certain
medications before elective surgery or procedures is to pre-
vent perioperative AKI and other complications such as hy-
potension or metabolic acidosis or hyperkalemia during the
perioperative period.
735
Medications that should be
discontinued before elective surgery due to potential
perioperative adverse effects are shown in Table 32.
735,736
There is consistent evidence that withholding RASi is
associated with lower risk of perioperative hypotension in
various types of surgery and procedures (noncardiac surgery,
cardiac surgery, and coronary angiography).
737,785,786
The
evidence that withholding RASi would lower perioperative
AKI is less consistent as affected by fewer studies with low
sample sizes.
787,788
In the surgical context,
antihyperglycemic agents such as sulfonylureas, metformin,
and SGLT2i would be held because of fasting before the
surgery. Case reports, case series, and a systematic review of
47 cases
739,740,789
support the current recommendations that
SGLT2i should be withheld at least 3–4 days before the
elective surgery.
741,742
Temporary discontinuation of medications to manage
adverse events is indicated in most cases. However, fear for
adverse event recurrence often results in failure to resume
treatments. In CKD, hyperkalemia or AKI are not uncommon
adverse effects of RASi treatment, to which clinical guidelines
recommend discontinuation of RASi and therapy reinitiation
at low dosages when the event is resolved.
23,29,790,791
Despite
this advice, permanent discontinuation of RASi seems to be
the most common clinical reaction to occur rence of adverse
events.
580,792
Observational studies consistently show that
withholding RASi medication compared with continuing
treatment after these adverse events is associated with a
lower recurrence of adverse events, but conversely a higher
risk of MACE and death, for which prevention is one of the
main indications for RASi.
502–506
See Section 3.11 on
hyperkalemia management.
Sick
Identies
dehydrating illness
Recalls or retrieves
list of pills to stop
Identies and
stops pills
Recovers and
resumes pills
Figure 47 | Essential steps for appropriate sick day rule implementation.
www.kidney-international.org chapter 4
Kidney International (2024) 105 (Suppl 4S), S117–S314 S251

In all these situations, enhanced communication with the
patients, and between inpatient and outpatient teams, is neces-
sary to ensure resumption of medications in a timely manner.
Special considerations
Pediatric considerations.
Many children with CKD with un-
derlying tubular disorders have an obligate urine output irre-
spective of their hydration status and are at particularly high risk
of hy potension and AKI during an acute dehydrating illness.
Therefore, temporary discontinuation of medications such as
diuretics and RASi that may lead to serious complications of
volume depletion, such as hypotension and AKI, should be
considered during illnesses. If medications are discontinued
during an illness, a clear plan of when to restart the discontinued
medications should be communicated to people with CKD and
documented in the medical record.
4.3.1 Strategies to promote drug stewardship
Practice Point 4.3.1.1: Educate and inform peo ple with
CKD regarding the expected benefits and possible risks of
medications so that they can identify and report adverse
events that can be managed.
People with kidney disease have a role in drug stewardship,
and given that they may receive medications from non-
nephrology healthcare providers, people with CKD should be
encouraged to inform those prescribers that they have kidney
disease to facilitate consideration of doses and potential side
effect of medications. Thus, education and information for
people with CKD inclusive for their population (i.e., literacy
level and languages) are encouraged. Although brochures and
conversations may be useful, interactive electronic health
applications have been shown to be acceptable to patients and
may lead them to apply the knowledge gained more effec-
tively.
793–797
Practical implementation tips involve printing
out the results of the most recent eGFR estimation for the
patient to bring along in future healthcare consultations and/
or write down a list of ongoing medications to alert other
healthcare providers of medication risks and benefits.
A diag nosis of CKD should always be reflected in medical
records, as this will alert physicians on the need to consider
adjusting or avoiding certain medications or procedures.
Under-recognition of CKD diagnoses in medical records is
associated with medication errors, includ ing potentially
inappropriate prescription of nephrotoxic medications.
729
Practice Point 4.3.1.2: Establish collaborative relationships
with other healthcare providers and pharmacists and/or
use tools to ensure and improve drug stewardship in people
with CKD to enhance ma nagement of their complex
medication regimens.
Clinical pharmacists are highly qualified experts in medi-
cines and, as part of the multidisciplinary team, can play a
pivotal role in improving the quality of care and ensuring
patient safety in a range of ways. This includes carrying out
structured medicatio n reviews for people with CKD and
associated health problems and improving patient safety,
outcomes, and value through a person-centered approach.
798–
800
In addition and where clinical pharmacists are not avail-
able, clinical decision support systems can optimize this
process through automation and decision support integrated
into the EMRs, supporting drug stewardship through alerts to
healthcare providers on the need for dose adjustment to
prevent adverse effects. In RCTs enrolling peop le with CKD,
electronic clinical decision support systems have demon-
strated efficacy in reducing medication errors, avoiding drug-
drug interactions, and improving dose adjustment of medi-
cations excreted by the kidneys.
801–806
Recognizing that many
of these tools may not be available in all communities, the
concepts of regular review and evaluation of medications by a
knowledgeable healthcare provider are a critical component
of care for people with CKD.
Special considerations
Pediatric considerations.
Parents and carers should be cen-
tral to drug stewardship for children with CKD, with
increasing involvement from the young person as they move
toward transition.
4.4 Imaging studies
Practice Point 4.4.1: Consider the indication for imaging
studies in accordance with general population indications.
Risks and benefits of imaging studies should be determined
on an individual basis in the context of their CKD.
The use of iodinated radiocontrast media has been asso-
ciated with the occurrence of AKI, with varying rates reported
in observational studies depending on the population studied,
the type, route and dose of agent being used, and the defi-
nition of nephrotoxicity. The term “contrast-induced AKI”
has been traditionally coined to describe this condition,
807
but
subsequent research characterizing this entity suggests causal
links to be weak,
807–809
and the term “contrast-associated AKI
(CA-AKI)” has been suggested instead.
Although there is potential risk for AKI with contrast
administration in people with CKD G4–G5, caution should be
exercised in withholding contrast treatment or evaluation of a
potentially fatal condition solely based on GFR.
810,811
Harm
Table 32 | Medications that should be considered for
temporary discontinuation before elective surgeries and
potential perioperative adverse events associated with their
continued use
Medications Potential perioperative adverse events
ACEi/ARB Hypotension, AKI
Diuretics Volume depletion, AKI
SGLT2i Ketoacidosis (starvation or diabetes)
Metformin Lactic acidosis if AKI occurs
Aminoglycosides Acute tubular necrosis/AKI
NSAIDs AKI, acute interstitial nephritis (AIN)
ACEi, angiotensin-converting enzyme inhibitor; AKI, acute kidney injury; ARB,
angiotensin II receptor blocker; NSAID, nonsteroidal anti-inflammatory drug; SGLT2i,
sodium glucose cotransporter-2 inhibitor.
chapter 4 www.kidney-international.org
S252
Kidney International (2024) 105 (Suppl 4S), S117–S314

may be induced through delaying, not performing, or
providing suboptimal diagnostic/therapeutic imaging
procedures due to fear of contrast-associated complications
to people with reduced GFR.
811,812
Tabl e 33
813
describes the
potential causes of CA-AKI identified in available studies that
may suggest an approach to people with CKD.
4.4.1 Radiocontrast: intra-arterial and intravenous dye studies
Practice Point 4.4.1.1: Assess the risk for AKI in people
with CKD receiving intra-arterial contrast for cardiac
procedures using validated tools.
The reported risk of CA-AKI is higher with procedures
involving arterial administration compared with venous
administration of contrast.
814
This difference in risk may be
due to differences in patient populations (those who require
arterial contrast are likely to have comorbidities that
increase the likelihood of AKI) or to differences in the
nephrotoxicity of intra-arterial contrast material.
Known risk factors for CA-AKI are advanced age, heart
failure, the volume of contrast material, proteinur ia, hyper-
glycemia, and use of RASi.
815
The highest risk for AKI is
associated with interventional (rather than diagnostic)
coronary angiography (particularly in the setting of acute
myocardial infarction). This may relate to the higher
volume of contrast used in interventional procedures and
hemodynamic instability associated with the clinical
situation.
815–817
Practice Point 4.4.1.2: The intravenous administration of
radiocontrast media can be managed in accordance with
consensus statements from the radiol ogy societies in people
with AKI or GFR <60 ml/min per 1.73 m
2
(CKD G3a–G5)
undergoing elective investigation.
The Work Group refers the reader to the most recent
radiology guidelines specifically noting the difference between
intravenous and intra-arter ial radiocontrast:
Use of low-osmolality contrast media and iso-osmolarity
contrast media
Use of minimum radiocontrast dose to achieve a diagnostic
study
Withdrawal of nonessential potentially nephrotoxic medi-
cations (e.g., NSAIDs, diuretics, aminoglycosides, ampho-
tericin, platins, zoledronate, and methotrexate) in people
with AKI or eGFR <30 ml/min per 1.73 m
2
for 24–48
hours before and 48 hours after radiocontrast exposure
In people with eGFR >30 ml/min per 1.73 m
2
and without
evidence of AKI, metformin need not be stopped before
iodinated contrast media (ICM) administration, and there
is no need for testing to evaluate GFR afterward. For people
with AKI or an eGFR #30 ml/min per 1.73 m
2
, it remains
appropriate to stop metformin at the time of or before ICM
injection and should not be restarted for at least 48 hours
and only then if GFR remains stable and the ongoing use of
metformin has been reassessed by the clinical team.
818
Given the lack of strong evidence demonstrating that
continuing RA ASi is benefici al, referring healthcare pro-
viders should consider withholding RAASi in people at risk
for $48 hours before elective contrast-enhanced CT to
avoid the potential for hypotension and hyperkalemia
should CA-AKI develop. RAASi may be restarted if CA-AKI
does not occur or after the return of GFR to baseline.
Consideration of avoiding dehydration for people not un-
dergoing dialysis and who have eGFR <30 ml/min per 1.73
m
2
or AKI and receiving intravenous contrast.
815,819
Use of N-acetylcysteine, ascorbic acid, furosemide, dopa-
mine, fenoldopam, or calcium channel blockers as pre-
ventative measures of CA-AKI has not been shown to be a
consistent benefit.
813
Prophylactic pericontrast hemodialysis has been shown to
be potentially harmful and is not recommended.
813
Special considerations
Global access to contrast agents.
The preference of contrast
agent may depend on availability and cost, particularly in
lower-income countries and lower-middle–income countries.
4.4.2 Gadolinium-containing contrast media
Gadolinium chelates used during magnetic resonance imag-
ing has previously been reported to cause nephrogenic sys-
temic fibrosis (NSF) before 2010, and the mechanisms have
been articulated.
820
Note that incidence of this condition has
not been reported later than 2012, thus raising the question as
to the true risk of this condition.
821
Practice Point 4.4.2.1: For people with GFR <30 ml/min
per 1.73 m
2
(CKD G4–G5) who require gadolinium-con-
taining contrast media, preferentially offer them American
College of Radiology group II and III gadolinium-based
contrast agents.
People who are at greatest risk for NSF include those with
AKI, undergoing KRT, and those with CKD G4–G5. Most
unconfounded cases have been associated with American
College of Radiology group I gadolinium-based contrast
Table 33 | Potential risk factors for contrast-associated acute
kidney injury
Patient-associated Procedure-associated
Reduced GFR, acute or chronic
a
High-osmolar contrast
Diabetes mellitus
b
Large volume of contrast
Reduced intravascular volume Serial contrast procedures
Concomitant nephrotoxic medications Intra-arterial procedures
GFR, glomerular filtration rate.
a
Defined as estimated glomerular filtration rate <45 ml/min per 1.73 m
2
with other
risk factors or eGFR <30 ml/min per 1.73 m
2
.
b
Augments risk in people with underlying kidney function impairment.
Reproduced from Cashion W, Weisbord SD. Radiographic contrast media and
the kidney. Clinical Journal of the American Society of Nephrology, volume 17, issue 8,
pages 1234–1242.
813
https://journals.lww.com/cjasn/fulltext/2022/08000/radiographic_
contrast_media_and_the_kidney.20.aspx. Copyright ª 2022 by the American Society
of Nephrology. The Creative Commons license does not apply to this content. Use
of the material in any format is prohibited without written permission from the
publisher, Wolters Kluwer Health, Inc. Please contact permissions@lww.com for
further information.
www.kidney-international.org chapter 4
Kidney International (2024) 105 (Suppl 4S), S117–S314 S253

media (e.g., gadodiamide, gadopentetate dimeglumine, and
gadoversetamide), and there is additional risk with repeated
doses.
822,823
Hence, in people with GFR <30 ml/min per 1.73 m
2
, the
use of newer linear and macrocyclic gadolinium-based
contrast media such as gadobenate dimeglumine, gadobutrol,
gadoterido l, gadoterate meglumine, and gadoxetate disodium
should be preferred.
824,825
Special considerations
Global access to gadolinium-contrast agents.
There are cost
implications in lower-income countries and lower-middle–
income countries as the nonlinear-chelated preparations are
more expensive.
Pediatric considerations. Considerations speci fic to the use of
gadolinium preparations in young children and neonates must
also be contemplated in addition to the general admonishments
against their use in situations of GFR <30 ml/min per 1.73 m
2
.
In particular, the FDA currently does not license any gadolin-
ium-based contrast media product for use in children <2 years
of age and, likewise, the European Medicines Agency cautions
against the use of any gadolinium-based contrast agents in a
child <1 year of age. The risk of NSF in pediatric populations
appears to be low, but data are limited.
822,823
In recognition of the inability to accurately measure GFR
in the neonate and, by extension, the clearance of compounds
such as gadolinium, all nephrologists and radiologists must
exercise caution in terms of use of gadolinium-based contrast
media in this potentially high-risk population, and all other
imaging modalities should be considered before choosing one
requiring gadolinium exposure. Although not based on spe-
cific evidence, some have suggested the avoidance of high-risk
gadolinium agents in very young children (e.g., neonates
younger than 4 weeks of age).
826
Moreover because of kidne y im maturity in fetu ses, neo-
nates, and infants, this population (and consequently preg-
nant women because of the risk to the fetus) is considered
potentially at r isk for NSF.
827
However, although the data are
limited, the number of reported cases of NSF in the pediatric
population is lower than in the adult population.
828
There
is no convincing evidence that pediatric populations have
anincreasedriskcomparedwithadults.TheriskofNSF
in pediatr ic patients appears to be low, but data are
limited.
823
For research recommendations, please see Chapter
6: Research recommendations.
chapter 4 www.kidney-international.org
S254
Kidney International (2024) 105 (Suppl 4S), S117–S314

Chapter 5: Optimal models of care
5.1 Referral to specialist kidney care services
Early identification and referral to specialist kidney care ser-
vices for people with CKD has the potential to reverse, delay,
or prevent progression of disease and is a key focus of in-
ternational initiatives in the context of the global “epidemic”
of kidney disease. The goals of early identification and referral
to specialist kidney care ser vices are several-fold and include:
Ensur ing a specific diagnosis for CKD is sought, where
appropriate
Provision of specific therapy based on diagnosis
Slowing/arresting CKD progression
Evaluation and management of comorbid conditions
Prevention and management of CVD
Identification, prevention, and management of CKD-spe-
cific complications (e.g ., malnutrition, anemia, bone dis-
ease, and acidosis)
Planning and preparation for KRT (e.g., choice of modality,
access-placement and care, and preemptive transplantation)
Psychosocial support
Provision of conservative care and palliative care options
where required.
Practice Point 5.1.1: Refer adults with CKD to specialist
kidney care services in the circumstances listed in
Figure 48:
The scope of nephrology practice in cludes a wide var iety of
conditions, not only kidney failure but also acute and chronic
primary and systemic diseases involving individual elements
of the kidney, resistant hypertension, and biochemical de-
rangements. Thus, there are many potential benefits of
nephrology referral in addition to those more commonly
recognized such as identification of reversible causes of CKD,
provision of treatment to slow progression of CKD, man-
agement of the metabolic complications of CKD, and prep-
aration for dialysis and transplantation.
Central to achieving the best outcomes for people with
CKD regardless of the reason for referral is timeliness.
Further evaluation and
specialist management
based on diagnosis
Causes
Circumstances category Circumstance examples Actions
Diagnosis of CKD
eGFR/risk of KRT
Planning and preparation
for kidney replacement
therapy
Albuminuria
and microscopic
hematuria
Further evaluation
and management
Others
Management of CKD
complications
• Cause of CKD is uncertain
• Hereditary kidney disease
• Recurrent extensive nephrolithiasis
• A >3%–5% 5-year risk of requiring KRT measured
using a validated risk equation
• eGFR <30 ml/min per 1.73 m
2
• A sustained fall in GFR of >20% or >30% in those
people initiating hemodynamically active therapies
• Consistent nding of signicant albuminuria
(ACR ≥300 mg/g [≥30 mg/mmol] or AER ≥300 mg/24 hours,
approximately equivalent to PCR ≥500 mg/g [≥50 mg/mmol]
or PER ≥500 mg/24 h) in combination with hematuria
• ≥2-fold increase in albuminuria in people with signicant
albuminuria undergoing monitoring
• A consistent nding of ACR >700 mg/g [>70 mg/mmol]
• Urinary red cell casts, RBC >20 per high power eld
sustained and not readily explained
• CKD and hypertension refractory to treatment
≥4 antihypertensive agents
• Persistent abnormalities of serum potassium
• Acidosis
• Anemia
• Bone disease
• Malnutrition
Figure 48 | Circumstances for referral to specialist kidney care services and goals of the referral. ACR, albumin-to-creatinine ratio; AER,
albumin excretion rate; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; KRT, kidney replacement therapy; PCR, protein-
creatinine ratio; PER, protein excretion rate; RBC, red blood cells.
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S255

Referral to specialists for assessment does not necessarily
equate to access to or need for multidisciplinary care, and
differentiation of the value of each is important. Specialist
referral to aid in ascertainment of cause and prognosis can be
seen as separate from care and support services targeted at
complications, and delay of, and preparation for progressive
CKD. Application of risk prediction tools (Chapter 2) may aid
decision-making in terms of identifying those at risk of
progression and determining action thresholds for multidis-
ciplinary care and placement of access for KRT or referral to
transplantation. Current recommendations to use validated
risk equations to ascertain those at high probability of kidney
failure within 2 years should prompt actions that align with
provision of appropriate education activities, review of un-
derstanding, and decision-making, and prompting referrals to
other healthcare providers (e.g., vascular access surgeons,
transplant teams, etc.).
Risk-based referral was compared with guideline referral
criteria in a cross-sectional study from the UK.
829
Analysis
revealed that approximately 40% of patients classified as at
high risk of progression to kidney failure by KFRE (>3% by 5
years) were missed by guideline referral criteria. Moreover, a
model predicting the timing of clinical outcomes, validated in
a multicenter prospective cohort study of 1517 people aged
$65 years with eGFR 10–30 ml/min per 1.73 m
2
, showed
good performance for predicting the timing and occurrence
of KRT. Using this prediction model to guide referral for
vascular access preparation resulted in less unnecessary
arteriovenous fistula surgeries than using eGFR thresholds.
830
In this section, we consider the evidence relating to timely
referral for planning KRT in people with progressive CKD.
The literature concerning late referral has been remarkably
consistent with both clinical studies and narrative reviews
identifying several adverse consequences of late referral and
related benefits of early referral (Table 34).
Both individual and healthcare system factors are associ-
ated with late referral for KRT planning. A systematic review
of 18 studi es and physician surveys identified specific factors
responsible for late referral for KRT as shown in Table 35.
831
We encourage each nephrology program to explore factors
associated with late referral to improve referral patterns
appropriately.
People with kidney disease have never been randomized to
early or late referral to nephrology services, and the definition of
late referral in the published studies varies between 1 and 12
months before the start of KRT. Three months is probably less
than the absolute minimum amount of time required for
assessment, education, preparation for KRT, and creation of
access, but 3 months is the most frequently employed definition.
A systematic review of 40 studies showed that early referral
was associated with better clinical and biochemical outcomes
such as improvement in mortality at 3 and 5 years, decrease in
hospitalizations, better access to vascular access and KRT with
peritoneal dialysis, as well as improvements in BP, hemoglobin,
and serum albumin (Table 36).
832
A retrospective study of
105,219 patients (early referral 21,024 patients and late referral
84,195 patients) showed that early referral to nephrology care
was associated with slower progression of CKD as significantly
more patients in early referral group did not change their CKD
stage (65%–72.9% vs. 52%–64.6%, P < 0.05).
833
Local practice and resources will dictate local referral
practices. Regardless of the healthcare system, delay, or pre-
vention of progression of both CKD and its complications
will be of value to both individuals and healthcare systems.
Local organizations will determine the best methods of
communication and interaction between people with CKD,
kidney care specialists, the multidisciplinary team, and pri-
mar y care physicians.
Technology may be used to promote appropriate
nephrology referral. Embedding clinical practice guidelines
into clinical information systems may effectively create a
reminder system for primary care physicians. Clinical deci-
sion support systems could also improve referral criteria
adherence. The smartphone application, Nefroconsultor,
which uses KDIGO referral criteria was shown to increase the
rate of appropriate referral by 28.8%.
834
Table 34 | Benefits and consequences of early versus late referral
Consequences of late referral Benefits of early referral
Severe hypertension and fluid overload, and increased cardiovascular
comorbidity
Delay requirement for KRT, better management of CVD, and
comorbid conditions
Low prevalence of permanent access Reduced need for urgent dialysis using temporary access
Delayed referral for transplant Greater choice of treatment options and pre-emptive transplantation
Higher initial hospitalization rate Reduced hospital length of stay and costs
Higher 1-year mortality rate Lower 1-year mortality rate
Less choice of KRT modality Increased informed freedom of choice of KRT modality
Worse psychosocial adjustment and increase in DALYs Early access to psychosocial counseling and support for care partners
Malnutrition Improved nutritional status
CVD, cardiovascular disease; DALY, disability-adjusted life-year; KRT, kidney replacement therapy.
chapter 5 www.kidney-international.org
S256
Kidney International (2024) 105 (Suppl 4S), S117–S314

Implementation of referral guidelines will inevita bly lead
to an increased workload for specialist kidney care ser vices.
However, introduction of local initiatives in conjunction with
primary care providers can improve the appropriateness and
quality of the referral. A checklist for goal-directed care in
CKD should be considered. Local initiatives combined with
national policy and practice changes can lead to an
improvement in the outcomes for people with CKD regard-
less of the level of resources available.
Special considerations
Pediatric considerations.
Practice Point 5.1.2: Refer children and adolescents to
specialist kidney care services in the following
circumstances:
an ACR of 30 mg/g (3 mg/mmol) or a PCR of 200 mg/g
(20 mg/mmol) or more, confirmed on a repeat first
morning void sample, when well and not during
menstruation,
persistent hematuria,
any sustained decrease in eGFR,
hypertension ,
kidney outflow obstruction or anomalies of the kidney
and urinary tract,
known or suspected CKD, or
recurrent urinary tract infection.
Children with known or suspected CKD or who are at risk
of CKD (as outlined above) should be referred to specialist
care. This allows for timely investigations and diagnosis. Early
integration of children with CKD into nephrology services
will ensure optimal management of pediatric complications
of CKD (including growth restriction) and will promote ac-
cess to preemptive transplantation (the KRT of choice).
5.2 Symptoms in CKD
5.2.1 Prevalence and severity of symptoms
CKD confers a high burden of uremic symptoms that may be
under-recognized, underdiagnosed, and undertreated.
835
As
kidney disease progresses, affected people experience an
increasing burden of adverse uremic symptoms. These
symptoms can impair their health-related QoL (HRQoL) by
interfering with social relationships, financial instability, and
contributing to overall poor well-being.
836
Patient-reported
outcomes, including HRQoL and symptoms, are often
identified by people with CKD as more important to them
than clinical outcomes, such as survival.
837,838
A recent
systematic review of 126 patient-reported outcome studies
involving people with CKD G1–G5, not on KRT, identified the
most common symptoms experienced in terms of prevalence
and severity in this population (Figure 49).
839
The most
prevalent symptom reported in the CKD population not
on KRT was fatigue at 70% (95% CI: 60%–79%), whereas in
the identified control population without CKD, fatigue
prevalence was 34% (95% CI: 0%–70%). In terms of the
symptoms reported as the most severe, sexual dysfunction had
the highest severity score. This review also looked at
populations receiving dialysis and/or transplantation, allowing
for the comparison of prevalence and severity across
populations. This provides insight into symptoms that may be
attributable to changing or deteriorating kidney function and
may provide symptom targets for tracking in the care of
people with CKD, especially those with more advanced CKD,
such as CKD G5.
5.2.2 Identification and assessment of symptoms
Practice Point 5.2.2.1: Ask people with progressive CKD
about uremic symptoms (e.g., reduced appetite, nausea, and
level of fatigue/lethargy) at each consultation using a stan-
dardized validated assessment of uremic symptoms tool.
The identi fication and assessment of symptoms in people
with progressive CKD are important for highlighting changes
in clinical management,
840
redirecting treatment toward
patient-centered management, and may lead to discussion
about appropriate supportive care options.
838
Effective 2-
way communication and shared decision-making should be
key principles between healthcare providers and the people
they treat, allowing them to work in partnership to identify
symptom burden, possible treatment strategies, and person-
centered solutions.
835,839,841
In the past, it had been challenging to find an accepted
standardized approach to assess and report outcomes for
Table 36 | Outcomes examined in a systematic review by
Smart et al.
832
Outcomes
Relative risk comparing early
vs. late referral
Receive permanent vascular access RR: 3.22; 95% CI: 2.92–3.55
Initiation of KRT with peritoneal dialysis RR: 1.74; 95% CI: 1.64–1.84
Three-month mortality OR: 0.61; 95% CI: 0.55–0.67
Five-month mortality OR: 0.66; 95% CI: 0.60–0.71
Outcomes
Mean difference in early
vs. late referral
Initial hospitalization (d) 9.1; 95% CI: 10.92 to 7.32
Systolic blood pressure (mm Hg) 3.09; 95% CI: 5.23 to 0.95
Diastolic blood pressure (mm Hg) 1.64; 95% CI: 2.77 to 0.51
Hemoglobin (g/dl) 2.76; 95% CI: 2.53 to 2.99
Serum albumin (g/dl) 1.92; 95% CI: 1.83 to 2.01
CI, confidence interva l; KRT, kidney replacement therapy; OR, odds ratio; RR, relative
risk.
Table 35 | Factors associated with late referral for kidney
replacement therapy planning
Patient-related factors Healthcare system–related factors
Age
Race
Comorbid illness
Etiology of kidney disease
Noncompliance or
nonadherence
Socioeconomic status
Health insurance status
Type of referring physician
Type of referring center
Health system and/or physician rationing
Distance to dialysis center
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S257

those with CKD, and patient repor ts of their HRQoL are still
rarely routinely recorded, despite increasing recognition of
their importance.
841–843
In addition, many of the assessments
developed have been for people on dialysis, with little vali-
dation in CKD populations not on KRT. In 2019, Verberne
et al.
841
described an international standard set of outcome
measures for people with CKD, developed in conjunction
with people with very high–risk CKD G3–G5. Within this
standardized set of outcome measures, there are 4 domains,
with one of the domains targeting 6 patient-reported
outcomes for HRQoL (fatigue, pain, general HRQoL,
physical function, depression, and daily activity). To date,
there is no consensus on a single preferred patient-reported
outcome measure (PROM) instrument to be used to assess
these symptoms. However, 3 generic tools have been
recommended by the International Consortium for Health
Outcomes Measurement (ICHOM) (Table 37).
The Patient-Repor ted Outcomes Measurement Informa-
tion System (PROMIS) tool has been evaluated in adults and
children with CKD, evidencing sufficient validity and
reliability.
844–846
Further study is still needed to investigate its
optimal use in routine nephrology care.
5.2.3 Management of common symptoms for people with CKD
Practice Point 5.2.3.1: Use evidence-informed management
strategies to support people to live well with CKD and
improve the ir health-related quality of life.
The goal of effective symptom management in people
with CKD is to assist them to live better with kidney disease,
regardless of life expectancy, within a supportive care
framework.
838
Unpleasant symptoms, such as CKD-
associated pruritis and emotional/psychological distress,
often occur within symptom clusters and treating one
symptom may potentially alleviate other symptoms.
835
Developing treatment strateg ies can be challenging given
the complexities of managing CKD in different populations
and the variation in levels of evidence for managing the
different symptoms experienced, with many strategies
extrapolated from studies of treatments in the general
Decreased appetite
Prevalence 42%
Severity score: 19.8
Leg swelling
Prevalence 45%
Severity score: no data
Fatigue
Prevalence 70%
Severity score: 22.8
Shortness of breath
Prevalence 42%
Severity score: 15
Muscle cramps
Prevalence 46%
Severity score: no data
Heartburn
Prevalence 46%
Severity score: no data
Drowsiness
Prevalence 53%
Severity score: 22.5
Pain
Prevalence 53%
Severity score: 22.5
Poor mobility
Prevalence 56%
Severity score: 19
Bone/joint pain
Prevalence 55%
Severity score: no data
Poor sleep
Prevalence 49%
Severity score: 23.8
Sexual dysfunction
Prevalence 48%
Severity score: 56.4
Itching
Prevalence 46%
Severity score: 25
Figure 49 | Common symptoms, prevalence, and severity in people with chronic kidney disease. To aid comparison of symptom severity
scores across different outcome measures, all mean severity scores were converted to a 0–100 scale, where a higher score indicates greater
severity. Adapted from Fletcher BR, Damery S, Aiyegbusi OL, et al. Symptom burden and health-related quality of life in chronic kidney disease:
a global systematic review and meta-analysis. PLoS Med. 2022;19:e1003954.
839
Copyright ª 2022 Fletcher et al. This is an open access article
distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/).
Table 37 | Recommended patient-reported outcome measurement tools for use in people with CKD
PROM tool Comments
SF-36 version 2 Widely used and well validated in many populations. Requires a license fee.
RAND-36 Older version of the SF-36. Does not require a license fee. Only available in English and Arabic.
PROMIS and
PROMIS-29
Both short forms are based on extensive item banks. Available in paper and electronic versions. Well validated in general
population with validation in people with CKD showing good reliability and sufficient validity in both adults and pediatric
populations.
CKD, chronic kidney disease; PROM, Patient-Repor ted Outcomes; PROMIS, Patient-Reported Outcomes Measurement Information; SF-36, 36-item Short Form Health Survey.
Table developed with data from Verberne et al.,
841
Selewski et al.,
844
and van der Willik EM, van Breda F, van Jaarsveld BC, et al. Validity and reliability of Patient-Reported
Outcomes Measurement Information System (PROMIS) using computerized adaptive testing (CAT) in patients with advanced chronic kidney disease. Nephrol Dial Transplant.
2022;38:1158–1169.
845
chapter 5 www.kidney-international.org
S258
Kidney International (2024) 105 (Suppl 4S), S117–S314

population or people on hemodialysis. For example, sexual
dysfunction, a very common and one of the most severe
symptoms described by people with CKD, is fraught with
barriers in terms of research from agreement of definitions,
the stigma of sexual dysfunction, acknowledging the
distinction between sex and gender, discordance between
research priorities and patient priorities, and understanding
that there are variable responses to treatment in people
with CKD.
847
However, there has been some consensus
that there is sufficient eviden ce to support guidance for
some symptoms such as uremic pruritis, sleep disturbances,
pain, depression, and restless leg syndrome,
838
but future
research is needed to understand the determinants of
symptoms such as chronic pain and evaluation of
management strategies.
848
Table 38
838,840,849–871
provides an
overview of the most common symptoms in CKD.
Practice Point 5.2.3.2: Screen people with CKD G4–G5, aged
>65, poor growth (pediatrics), or symptoms such as invol-
untary weight loss, frailty, or poor appetite twice annually
for malnutrition using a validated assessment tool.
Practice Point 5.2.3.3: Enable availability of appropriate
medical nutrition therapy for people with signs of malnu-
trition, ideally under the supervision of renal dietitians or
accredited nutrition providers if not available.
Table 38 | Management strategies for common symptoms in CKD
Symptom Comment
Management strategies
Lifestyle Pharmacological Other
Pain Management should be
determined by etiology and
severity
Physiotherapy, exercise and
massage therapy, and heat
for musculoskeletal pain.
Consider complementary
therapies such as
acupuncture.
838,840,849
Use of an adapted World Health
Organization (WHO) Analgesic
Ladder that takes into account
pharmacokinetic data of analgesics
in CKD.
850
Before starting opioids, healthcare
providers should assess risk of
substance abuse and obtain
informed consent after a discussion
around goals, expectations, risks,
and alternatives.
Topical analgesics may be
effective but used with caution
to avoid adverse events due to
systemic absorption. There are
no studies on long-term use of
any analgesics in people with
CKD; therefore, attention should
be paid to issues of efficacy and
safety.
Referral to a specialist pain
clinic or palliative/
supportive care clinic may
be beneficial for those at risk
of aberrant behaviors,
adverse outcomes, or in
special circumstances such
as end of life.
849
Sleep disorders Associated with fatigue,
poor HRQoL.
838
May be
related to pruritus, pain,
anemia, anxiety/depression,
and shortness of breath.
840
Management of basic sleep
hygiene, exercise, optimal
positioning when sleeping,
and removal of dietary or
other stimulants
838
Melatonin
851
and simple
sedatives
852,853
Cognitive behavioral
therapy,
854
addressing
contributing factors such as
anemia, fluid retention,
mood disorders, pain, and
pruritus
Restless leg
syndrome
Associated with impaired
sleep and HRQoL
Management of basic sleep
hygiene, exercise, optimal
positioning when sleeping,
and removal of dietary or
other stimulants
838
Cessation of medications that
interfere with the dopamine
pathway, or trials with
levodopa, nonergot dopamine
antagonists, or low-dose
gabapentinoids
855-857
Correction of contributing
factors such as
hyperphosphatemia and
iron deficiency/anemia
Uremic pruritus Associated with decreased
HRQoL and contributes to
other symptoms, such as
poor sleep, fatigue, and
depression
838
Acupunture
858
Gabapentinoids with continued
assessment of symptom experience
and titration by a medical
provider
859-861
Topical agents (capsicum,
rehydrating emollients if concurrent
dry skin)
861
Ultraviolet B therapy
862
Topical cannabis can be
considered
863
(Continued on following page)
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S259

In different world regions, 11%–50% of adults and 20%–45%
of children with CKD have malnutrition characterized by pro-
tein energy wasting (PEW).
872–874
In a European cohort of 1334
adults over the age of 65 years w ith CKD G4–G5, 25% were
found to have moderate malnutrition, and the risk was increased
with advancing age, female gender, and psychiatric disease.
875
Malnutrition can happen at any stage of CKD and is associated
with a higher morbidity and mortality, loss of muscle mass,
and inflammation. It can also be associated with worse
outcomes with kidney transplant.
874
The risk of PEW increases
as CKD progresses but is also influenced by comorbid
conditions such as diabetes, autoimmune diseases, and CVD.
PEW is thought to be driven by the damaging effect of uremic
toxins on appetite and chronic inflammation.
873–875
Given the
impact on prognosis and QoL, nutritional assessment and
intervention (ideally by a renal dietitian or accredited nutrition
provider) using a validated assessment tool should be
undertaken for people with CKD who present with frailty, age
>65 years, weight loss, poor growth (pediatrics), poor
appetite, and all people with CKD G4 and G5 (Table 39
876–878
).
Table 38 | (Continued) Management strategies for common symptoms in CKD
Symptom Comment
Management strategies
Lifestyle Pharmacological Other
Depression May be related to CKD
burden and perception, loss
of control, and medication
effects.
Associated with increased
morbidity, hospitalization,
and mortality, and is integral
to the assessment of
HRQoL
838
Exercise
864
and
acupuncture
865
Before commencing
pharmacological treatment for
depression, healthcare
providers should be aware of the
potential necessity to adjust
dosage, and follow-up with the
patient, due to altered
pharmacokinetics in CKD.
840
In
some circumstances this may
need to be done in
conjunction with specialist
psychiatric services.
Options may include:
Serotonin reuptake inhibitors
(e.g., citalopram, escitalopram,
fluoxetine, paroxetine, and
sertraline)
Serotonin-norepinephrine reup-
take inhibitors (e.g., venlafaxine,
duloxetine, and mirtazapine)
Atypical antidepressants (e.g.,
bupropion, trazodone, and
nefazodone)
Tricyclic antidepressants (e.g.,
amitriptyline)
866-869
Cognitive behavioral
therapy
870
Social support
869
Address contributing factors
(e.g., pain, pruritus and
mood disorders)
Poor appetite and
anorexia
Associated with depression,
malnutrition, poor HRQoL,
increased hospitalization,
and mortality rates
838
Increased physical activity
may increase appetite
871
No data to support the use of
appetite stimulants in people with
CKD not on KRT.
Management has not been studied
systematically in CKD.
838
Address contributing
factors (pain, heartburn,
mood disorders, any
dental issues/mouth
ulceration, constipation,
social and economic
factors, and lack of
physical activity)
Dietary assessment by a
dietitian
Nausea and
vomiting
Impact has not been
assessed systematically in
CKD.
838
Pharmacological management has
not been systematically studied in
CKD.
838
Address contributing
factors (pain, heartburn,
mood disorders, any
dental issues/mouth
ulceration, constipation,
social and economic
factors, and lack of
physical activity)
Dietary assessment by a
dietitian
CKD, chronic kidney disease; HRQoL, health-related quality of life; G3, estimated glomerular filtration rate (eGFR) 30–59 ml/min per 1.73 m
2
; G5, eGFR <15 ml/min per 1.73 m
2
;
KRT, kidney replacement therapy.
Table adapt ed from Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing
a roadmap to improving quality care. Kidney Int. 2015;88:447–459.
838
ª 2015 International Society of Nephrology.
chapter 5 www.kidney-international.org
S260
Kidney International (2024) 105 (Suppl 4S), S117–S314

5.3 Team-based integrated care
Practice Point 5.3.1: Enable access to a patient-centered
multidisciplinary care team consisting of dietary coun-
seling, medication ma nagement, education, and counseling
about different KRT modalities, transplant options, dialysis
access surgery, and ethical, psychological, and social care
for people with CKD.
An optimal care model leads to the best outcomes for the
individual, the population, and the community. The model of
care varies according to CKD severity and risk of progression
to kidney failure, which will determine the target population
and goals (Figure 50).
CKD models of care follow the same principles embodied
in the chronic disease model of care (Figure 51
879
). Each key
component of the chronic care model is applied to the CKD
care model.
The specific components for CKD models of care are
presented in Figure 52 and include:
(i) Navigation system that leads to appropriate and
timely referral. This relies on a good healt hcare system
(ii) An education program that includes both general
CKD and KRT education, including conservative
management, where appropriate
(iii) Surveillance protocols for laboratory and clinic visits,
attention to cardiovascular comorbidities, and CKD-
associated comorbidities such as anemia, a vaccina-
tion program
(iv) Management that includes self-care management
particular ly lifest yle modifi cation includ ing diet,
Table 39 | List of validated assessment tools for malnutrition
Validated malnutrition assessment tool Attributes
7-Point Subjective Global
Assessment (SGA)
876
Provides assessment points on weight change, dietary intake, digestive function, functional capacity,
and metabolic stress. A nutrition focused physical examination is also performed. This updated version
of the SGA is more sensitive to short-term nutrition changes. A score of 1–2 indicates severe malnutrition,
3–5 is mild malnutrition, and 6–7 indicates normal nutrition status.
Malnutrition-Inflammation Score
877
Assesses malnutrition and inflammation using 10 parameters including dietary intake, anthropometric
measurements, laboratory indices, and functional capacity. The score ranges from 0 (normal) to
30 (severe malnutrition and inflammation).
Mini Nutrition Assessment
878
Includes assessment of dietary intake, mobility, neuropsychology, and some anthropometric measurements,
including weight and calf circumference. A score of 12–14 points indicates normal nutrition status,
8–11 indicates at risk for malnutrition, and 0–7 points indicates malnutrition.
KRT preparation team/
comprehensive conservative care team
Nephrologist,
multidisciplinary care team
Primary care
CKD G5
CKD G4
CKD G3
CKD G2
CKD G1
Preparing
for KRT
Increasing severity of CKD
CV risk reduction
Identify patients at high risk
Discuss
KRT options
Education
Promote
self-management
Prevention and management
of complications
Kidney failure risk
≥40% in 2 years
Kidney failure risk
≥10% in 2 years
Kidney failure risk
≥3%–5% in 5 years
Kidney failure risk
<3%–5% in 5 years
Figure 50 | Optimal care model by increasing severity of chronic kidney disease (CKD). CV, cardiovascular; KRT, kidney replacement
therapy.
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S261

exercise, and smoking cessation, as well as medica-
tions and psychosocial support for issues such as so-
cial bereavement, depression, and anxiet y
(v) Three-way communication between people with
CKD, their multidisciplinar y specialist care team, and
their primary care providers
There are various CKD care models around the world. The
key features of existing CKD care models described in sys-
tematic reviews are shown in Table 40.
32,880–882
Practice Point 5.3.2: Education programs that also involve
care partners where indicated are important to promote
informed, activated people with CKD.
An effective patient educa tion program is a critical success
factor of self-care management support strategies. Education
should address 3 main issues:
(i) standardized educational topics and resources,
(ii) strategy to provide education effectively, and
(iii) patient-centered concept.
The suggested components of effective patient education
programs are illustrated in Figure 53. Each should be tailored
Community
Resources
and policies
Self-
management
support
CKD health systems
Organization of CKD care
Delivery
system
design
Decision
support
Clinical
information
systems
Informed
activated patient
Prepared proactive
multidisciplinary team
Productive
interactions
Improved outcomes
Figure 51 | The chronic care model. The chronic care model
emphasizes the additive benefits of different components in the
system, policy, provider, and patient levels in improving clinical
outcomes. CKD, chronic kidney disease. Reproduced from Epping-
Jordan JE, Pruitt SD, Bengoa R, Wagner EH. Improving the quality of
health care for chronic conditions. BMJ Quality & Safety, volume 13,
pages 299–305, Copyright ª 2004, with permission from BMJ
Publishing Group Ltd.
879
Education
• To individual and family
• About disease, diet,
lifestyle, and medications
Navigation
• Disease triage
• Transition
• Healthcare system
Surveillance
• Symptoms
• Blood tests
• Urine tests
• Other investigations
Management
• Medications
• Psychological support
Figure 52 | Specific components of the chronic kidney disease model of care.
Table 40 | Key features of existing CKD care models
32,880-882
Multidisciplinary care team composition
Nephrologist
Endocrinologist, cardiologist,
transplant surgeon,
psychologist, etc.
Nurse
Pharmacist
Renal dietitian or accredited
nutrition provider
Social worker
Interventions
BP management
Diabetic management
Cardiovascular management
Anemia management
Mineral and bone disorder
management
Conservative kidney
management
Education on dialysis modality
selection
Vascular access planning
Transplantation education and
evaluation
Nutritional and dietary counseling
Medication reconciliation
Vaccination program
Outcomes
Delay progression of CKD
Improve BP control
Improve CVD outcomes
Improve rate of optimal medication
Improve patient education
ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker;
BP, blood pressure; CKD, chronic kidney disease; CVD, cardiovascular disease.
chapter 5 www.kidney-international.org
S262
Kidney International (2024) 105 (Suppl 4S), S117–S314

to individual needs, circumstances, and resources, see text for
details.
Standardized educational topics should cover 3 main
subject areas: knowledge about CKD, knowledge about
treatment to slow progression and complications of CKD, and
knowledge about the kidney failure management options.
Educational material should be written and explained
clearly with plain language. Customization of information to
patient needs and literacy level, and sensitive to cultural
norms and needs (i.e., storytelling/videos vs. written mate-
rials). A multidisciplinary approach should be encouraged as
an effective strategy for providing education. Engaging com-
munity healthcare workers and other health education
providers may be an effective strateg y for providing patient/
carer education and empowering self-care management.
883
Targeting education to people w ith CKD who are at high
risk of CKD progression might y ield a better outcome than
routine care not only to the individual but also to the
healthcare system. Engaging with family members or
caregivers in a CKD education program will facilitate self-
care management and psychosocial support.
Practice Point 5.3.3: Consider the use of telehealth tech-
nologies including web-based, mobile applications, virtual
visiting, and wearable devices in the delivery of education
and care.
1. Create standardized educational material
• Cover pertinent information relevant to patient needs
• Use culturally and linguistically appropriate materials
• Available in various formats
Knowledge about CKD
• Kidney anatomy and function
• Type of kidney failure
• Etiology of CKD
• Signs and symptoms of CKD
• Kidney tests
Knowledge about CKD management
• Treatment to slow progression of CKD
• Treatment of CKD complications
• Medication commonly used in people
with CKD
• Medication safety in people with CKD
• Sick day rules
• Lifestyle modication
• Nutritional therapy
Knowledge about
treatment options in kidney failure
• Comprehensive conservative treatment
• Peritoneal dialysis
• Hemodialysis
• Kidney transplantation
Suggested educational topics*:
Target group:
CKD G1–G2 CKD G3–G4 CKD G5
2. Strategy to deliver educational session
• Multidisciplinary care team approach
• Community health care network
• Involving primary care physician
• Patient-to-patient discussion or peer support groups
• Focus on improvement of patient's health literacy
3. Patient-centered
• Target high-risk people with CKD
• Triage patient to specic educational topics they need
• Engage family members and/or caregivers
Key objectives:
Improving
CKD awareness
Choose
appropriate
KRT modality
• Understand how to prevent CKD progression
and management of CKD complications
• Empower self-management
Figure 53 | Strategy for effective patient education programs for people with chronic kidney disease (CKD). *Should be tailored to
individual needs and wishes. KRT, kidney replacement therapy.
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S263

Telehealth has been used increasingly in medicine,
including nephrology, during the COVID-19 pandemic.
Telehealth has the potential to augment patient care in CKD
in many aspects such as improving access to CKD care in
outreach patients, increasing patient monitoring ability,
helping with healthcare provider shortage, and improving
patient satisfaction. Telehealth in nephrology (“Tele-
nephrology”) can be categorized into 3 main areas: (i) remote
monitoring, (ii) providing education, and (iii) deliver y of
care. These have been implemented in 4 main platforms
including internet web-based, smartphone applications,
interactive video conferencing, and wearable technology.
Remote monitoring technology has been designed to
promote self-care management through oversight of clinical
parameters so people with CKD can monitor changes at
home, such as BP, body weight, or abnormal symptoms.
884,885
This may encourage people with CKD to participate in the
management of CKD.
Telehealth technologies that enhance education in people
with CKD have been reported in various forms. Web-based
applications are probably the most popular platform used to
provide education for people with CKD and their families.
886
Systematic reviews suggest that web-based CKD materials are
mostly adequate but not written at a suitable literacy level for
most people with CKD.
887,888
Smart phone applications have been increasingly adopted
for patient education in CKD. Educational material can be
installed into smartphone applications as a tool for on-de-
mand knowledge. Moreover, smartphones applications that
provide self-care management support for people with CKD
were reported in a pilot study.
889
The application targeted 4
key self-care management parameters: monitoring BP,
medication management, symptom assessment, and
tracking laboratory results. Lastly, interactive video
conferencing can provide patient education simultaneously
with a virtual visit.
890,891
This strategy should not be
intended to replace the clinic visit but would be helpful for
dealing with any event that happens between follow-up
face-to-face visits, such as follow-up of clinical symptoms
after starting or adjusting medication. Examples of
telehealth technologies that were studied in people with
CKD are shown in Figure 54.
Standardized and culturally appropriate protocols should
be considered. Although it is recognized that resources may
vary across and within jurisdictions, the recommendations
here are based on principles of care, which should be relevant
across the globe.
CKD is a complex condition that coexists with many other
conditions. Therefore, models of care should be developed
that integrate the complexity of the clinical conditions
involved, patient-centered philosophi es, and the healthcare
environment. The principles of care are universal, but
implementation may be customized to specific circumstances.
Special considerations
Pediatric considerations.
5.3.1 Transition from pediatric to adult care
5.3.1.1 Pediatric providers
Practice Point 5.3.1.1.1: Prepare adolescents and their
families for transfer to adult-oriented care starting at 11–14
years of age by using checklists to assess readiness and
guide preparation, and by conducting part of each visit
without the parent/guardian present (Figure 55).
Practice Point 5.3.1.1. 2: Provide a comprehensive written
transfer summary, and ideally an oral handover, to the
receiving healthcare providers including all relevant med-
ical information as well as information about the young
person’s cognitive abilities and social support (Figure 55).
Practice Point 5.3.1.1.3: Transfer young people to adult care
during times of medical and social stability where possible.
Although several organizations have made recommendations
about transition from pediatric to adult care, there have been no
randomized trials to test the effectiveness of specificap-
proaches.
892–894
Nevertheless, there is general agreement that
preparation for transfer to adult care should start as early as 11
years of age and certainly by 14 yearswhenpossible.
895
Anumber
of tools are available to guide preparation. Checklists to assess
CKD care aspects Domains Platforms
Remote monitoring
Education strategies
Delivery of care
Asynchronous
Synchronous
Asynchronous
Synchronous
Asynchronous
Synchronous
Wearable technology
Web-based with interactive application
Web-based and smartphone applications
Interactive video conferencing
Smartphone applications
Interactive video conferencing
Telehealth
Figure 54 | Telehealth technologies for people with chronic kidney disease (CKD).
chapter 5 www.kidney-international.org
S264
Kidney International (2024) 105 (Suppl 4S), S117–S314

readiness (i.e., TRxANSITION, Youth Quiz from the On Trac
program, Transition Readiness Assessment Questionnaire
(TRAQ), Readiness for Transition Questionnaire (RTQ), and
Got Transition tools http://www.gottransition.org) are useful
to identify the areas of weakness.
896–899
Young people should
gradually be prepared for full autonomy with medical visits.
Seeing the young person alone before inviting caregivers into
the room allows young people to practice interacting with
healthcare providers independently and provides privacy for
the discussion of sensitive topics.
Good communication between the transferring and
receiving care teams is a cornerstone of successful transitions.
A comprehensive written medical summary must be provided;
a verbal handover is ideal. Because childhood CKD may be
associated with neurodevelopmental disabilities, a clear
description of the young person’s cognitive abilities, including
strengths and weaknesses that may influence their ability for
self-care management, is critical. Information about social
support available to young people is also important.
Healthcare transitions are well known to be strongly
associated with adverse outcomes, including loss to follow-up.
Transferring dur ing periods of instability is ill-advised and
may amplify the risk of poor outcomes.
894
To minimize the
risk of loss to follow-up, pediatric care providers should
follow-up with patients to ensure that they have engaged
with the new care team.
Transition clinics may improve the outcomes of young
people transitioning from pediatric to adult care.
900,901
Transition clinics may be staffed exclusively by pediatric
care providers and focus on preparation, or may be jointly
staffed by pediatric and adult providers.
895,902
Although
joint pediatric-adult clinics are viewed as ideal, their
superiority has not been demonstrated in randomized trials.
Furthermore, feasibility may be limited by funding,
geography, and staffing. Young people should have the
opportunity to visit the adult clinic before transfer.
5.3.1.2 Adult providers
Practice Point 5.3.1.2.1: Recognize that young people under
25 years of age with CKD are a unique population at high
risk for adverse outcomes at least in part due to physiologic
incomplete brain matura tion.
Practice Point 5.3.1.2.2: Encourage young people to infor-
mally visit the adult care clinic to which they will be
transferred before the first appointment (Figure 55).
Practice Point 5.3.1.2.3: Assess young people with CKD
more frequently than older people with the same stage of
CKD and, with the agreement of the young person, include
the caregivers or significant other of the young person in
their care, at least in the first 1–3 years following transfer
from pediatric care (Figure 55).
Even for young people without chronic illness, the interval
between 14 and 25 years of age is a period of change and
increasing autonomy. Young people with CKD undergoing
transfer to adult care must navigate 2 transitions simulta-
neously: the transitio n of care and the larger transition from
childhood to adulthood. Development of the prefrontal cor-
tex, responsible for planning, organization, and impulse
control, continues to approximately 25 years of age. Adult
care providers must recognize that young adults constitute a
high-risk population requiring special care.
903
Outcomes are
poorer during this interval than at other times of life.
904
Care must reflect the fact that this is a high-risk period.
An informal visit to the new clinic setting may help in
reducing stress, improving engagement, and reducing loss to
follow-up.
895
In the initial years after transfer, visits should be
more frequent than for older adults with the same stage of
CKD to provide an opportunity for care providers to establish
a relationship with the young person, reduce the risk of loss to
follow-up, improve adherence to medications, and provide
Transition
Preparation for transfer
Pediatric care
Preparation for regular adult care
Joint pediatric-adult care
or
Young adult care
Transition
Regular
adult care
Transfer – when stable
• Allow young people to visit the clinic before transfer
• Recognize that “emerging adulthood” is a period of
high risk for adverse outcomes
• See emerging adults more frequently than older adults
with same stage of CKD
• Include caregivers or signicant others in patient visits,
with permission of patient
Age (years)
11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26
• Start early (11–14 yr)
• Use checklists to assess readiness
and guide preparation
• See young person alone for
at least part of each visit
• Comprehensive written
summary and verbal
handover, including
cognitive ability and
social support
• Follow-up after transfer
Figure 55 | The process of transition from pediatric to adult care in chronic kidney disease (CKD).
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S265

enhanced monitoring of a group at high risk of adverse
outcomes. Although young adults must have an opportunity
to meet their care providers alone, many will continue to
desire and need involvement of parents or significant others in
their care. This is a normal part of development, is associated
with better outcomes, and should be encouraged.
895
Multidisciplinary young adult clinics including youth
workers, social workers, pharmacists, and psychologists in
addition to physicians and nurses may be beneficial.
900
Peer-
support programs have also shown promise.
904
5.4 Timing the initiation of dialysis
Practice Point 5.4.1: Initiate dialysis based on a composite
assessment of a person’s symptoms, signs, QoL, prefer-
ences, level of GFR, and laboratory abnormalities.
Practice Point 5.4.2: Initiate dialysis if the presence of one
or more of the following situations is evident (Table 41).
This often but not invariably occurs in the GFR range
between 5 and 10 ml/min per 1.73 m
2
.
Practice Point 5.4.3: Consider planning for preemptive
kidney transplantation and/or dialysis access in adults
when the GFR is <15– 20 ml/min per 1.73 m
2
or risk of
KRT is >40% over 2 years.
These statements are worded very precisely to highlight the
need to plan proactively for complex activities related to
initiation of KRT. Also, there is a need to address symptoms
and to avoid the institution of dialysis therapy at an arbitrary
number representing the degree of residual kidney function.
Given the risks and benefits of KRT, as well as the potential
imprecision of measurements, people with CKD need to be
treated according to symptoms and signs, not simply bas ed
on laboratory values. Data from the Initiating Dialysis Early
and Late (ID EAL) RCT show no survival advantage to early
start dialysis (i.e., at higher levels of GFR).
905
Thus, the
statement as written should help the healthcare provider to
balance symptoms with laboratory values in decision-making.
Secondary analyses of the IDEAL study showed no signifi-
cant difference in QoL or healthcare–related cost between early
and late star t dialysis groups.
905,906
Moreover, subgroup
analysis of the IDEAL study revealed no benefits on cardiac
outcome in the early-start dialysis group.
907
Since the IDEAL
study, there were a number of large sample size observational
studies with an advanced statistical technique to reduce
possible confounding factors and biases encountered in
previous observational studies.
908–910
The overall results were
consistent with the IDEAL study and showed no benefits of
early-start dialysis compared with late-start dialysis in regard
to mortality and hospitalization risk (Table 42
905–909
).
Factors such as availability of resources, reasons for starting
dialysis, timing of dialysis initiation, patient education and
preparedness, dialysis modality and access, as well as varied
“country-specific” factors significantly affect a person’s expe-
riences and outcomes. As the burden of kidney failure
has increased globally, there has also been a growing recogni-
tion of the importance of patient involvement in determining
the goals of care and decisions regarding treatment. It is
important to move away from a “one-size-fits-all” approach to
dialysis and provide more individualized or personalized care.
The availability of resources for formal multidisciplinary
teams, educational materials, and accessto specializedcounseling
for diet, advance directives, access planning, and preemptive
transplantation varies around the world. These statements are
proposed so that “best practices” can be documented or aspired
to. The need for education, planning, and appropriate expertise
for the management of this patient group is internationally
relevant. The methods, frequency, and tools with which this can
be accomplished will be region specific.
There is a need to focus on regular symptom assessment as
part of the CKD review in those w ith lower eGFR values.
Individual assessment and availability of resources will dictate
specific timing of therapies. Healthcare providers should be
aware of the impact of early dialysis start on QoL before
recommending this strategy to people with CKD.
Recognition that the planning of smooth transition to
either dialysis or transplantation from advanced CKD re-
quires alignment of multiple different resources and activities,
as such the planning for these will be situation specific. It is
important to recognize that there is variability in the avail-
ability of vascular access serv ices or peritoneal dialysis cath-
eter insertion for those who choose hemodialysis or
peritoneal dialysis, respectively, and access to preemptive
transplantation. The complexity of the decision-making and
different teams and resources required to effectively transition
people often requires time, and thus the recommendation to
begin “planning of KRT” is intentionally advised at a con-
servative time point.
Special considerations
Pediatric considerations.
Practice Point 5.4.4: In children, in addition to the adult
indications for dialysis, poor growth refractory to opti-
mized nu trition, growth hormone, and medical manag e-
ment is an indication for initiating KRT.
Table 41 | Indications for the initiation of dialysis
Symptoms or signs attributable to kidney failure (e.g., neurological signs and symptoms attributable to uremia, pericarditis, anorexia, medically resistant
acid-based or electrolyte abnormalities, intractable pruritus, serositis, and acid-base or electrolyte abnormalities)
Inability to control volume status or blood pressure
Progressive deterioration in nutritional status refractory to dietary intervention, or cognitive impairment
chapter 5 www.kidney-international.org
S266
Kidney International (2024) 105 (Suppl 4S), S117–S314

Practice Point 5.4.5: Pursue living or deceased donor pre-
emptive kidney transplantation as the treatment of choice
for children in whom there is evidence of progressive and
irreversible CKD. The eGFR at which preemptive trans-
plantation should be undertaken will depend on multiple
factors including the age and size of the child and the rate
of progression of kidney failure but will usually be between
eGFR 5–15 ml/min per 1.73 m
2
.
In children, poor growth can also be a reason to initiate
dialysis. The decision to start dialysis should be reached in
discussion with the child (if age appropriate), their caregivers,
and their healthcare providers. Medical and psychosocial
preparations for the initiation of dialysis should begin well
before dialysis is required.
Deferred initiation should not imply deferred preparation,
and early discussions regarding medical and psychosocial
preparation for the initiation of dialysis should not be delayed
(e.g., placement of dialysis access, dialysis modality
selection, advance care planning, and assistance with home
therapies).
In children, studies from the USRDS, the European Society
of Paediatric Nephrology (ESPN), and the Australia and New
Zealand Dialysis and Transplant Registry (ANZDATA) found
no benefit from starting dialysis early.
911–913
Of 15,000 inci-
dent children on dialysis in the USRDS, the mortality risk was
36% higher for those with eGFR >10 ml/min per 1.73 m
2
compared with those with lower eGFR at dialysis initiation.
912
Mortality risk increased in those starting dialysis with eGFR
<5 and $12 ml/min per 1.73 m
2
, with a greater risk in
people 6 years and older.
914
A retrospective ESPN study of
nearly 3000 children found that mortality did not differ
when dialysis was started with an eGFR above or below 8
ml/min per 1.73 m
2
.
911
These observational data may be
confounded by indication bias.
Table 42 | Studies examining the timing of dialysis in people with CKD
Study Study design Comparison/study populations Outcomes Results
Cooper et al.
2010: IDEAL
study
905
RCT Late start group (eGFR
CG
5–7 ml/min
per 1.73 m
2
)
Early start group (eGFR
CG
10–14 ml/min
per 1.73 m
2
)
Mortality HR with early initiation, 1.04; 95% CI: 0.83–1.30;
P ¼ 0.75
Harris et al.
2011
906
Post hoc analysis
of IDEAL study
Late start group (eGFR
CG
5–7 ml/min
per 1.73 m
2
)
Early start group (eGFR
CG
10–14 ml/min
per 1.73 m
2
)
Cost
Quality of life
No statistical difference between early start vs.
late start group
Whalley et al.
2013
907
Post hoc analysis
of IDEAL study
Late start group (eGFR
CG
5–7 ml/min
per 1.73 m
2
)
Early start group (eGFR
CG
10–14 ml/min
per 1.73 m
2
)
Change in cardiac
structure and
function (LVMI, LVEF,
LAVI) over 12 months
and between groups
No statistically significant change in cardiac
structure and function over 12-month follow-
up. No statistically significant difference in
cardiac structure and function between the 2
groups
Rosansky et al.
2011
910
Observational
study
81,176 subjects with kidney failure
aged 20– 64 years, without diabetes,
and with no comorbidity other than
hypertension
1-year mortality The unadjusted 1-year mortality by MDRD
eGFR at dialysis initiation ranged from 6.8% in
the reference group (eGFR <5.0 ml/min per
1.73 m
2
) to 20.1% in the highest eGFR group
($15.0 ml/min per 1.73 m
2
).
Nacak et al.
2016
909
Observational
study
35,665 subjects with serum albumin
concentrations of 3.5 g/dl or higher
before hemodialysis initiation
1-year mortality 1-year mortality was 4.7%. In this group, the
adjusted HR for mortality was 1.27 for eGFR
5.0–9.9 ml/min per 1.73 m
2
, 1.53 for eGFR
10.0–14.9 ml/min per 1.73 m
2
, and 2.18 for
GFR $15.0 ml/min per 1.73 m
2
compared with
the reference group of GFR <5.0 ml/min per
1.73 m
2
.
Fu et al.
2021
908
Observational
study
10,290 people with CKD G4–G5;
compare dialysis initiation strategies
with eGFR values ranging between 4
and 19 ml/min per 1.73 m
2
and use an
eGFR between 6 and 7 ml/min per 1.73
m
2
as the reference group
5-year mortality The maximum 5-year mortality risk reductions
were 5.1% (for eGFR
15-16
vs. eGFR
6-7
),
translating into a better survival of only 1.6
months over a 5-year period at the expense of
starting dialysis 4 years earlier
CG, Cockcroft-Gault; CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; IDEAL, Initiating Dialysis Early and Late;
LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MDRD, Modification of Diet in Renal Disease; RCT, randomized controlled
trial.
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S267

5.5 Structure and process of supportive care and
comprehensive conservative management
Practice Point 5.5.1: Inform people with CKD about the
options for KRT and comprehensive conservative care.
Practice Point 5.5.2: Support comprehensive conservative
management as an option for people who choose not to
pursue KRT.
Practice Point 5.5.3: Provide access to resources that enable
the delivery of advanc ed care planni ng for people with a
recognized need for end-of-life care, including those people
undergoing comprehensive conservative care.
These statements are intended to highlight the importance
of supportive care and the need for comprehensive conser-
vative care processes and resources in the care of this complex
patient group. The term supportive care in nephrology means
care that is focused on improving the HRQoL for people with
CKD at any severity or age and can be provided along with
therapies intended to prolong life, such as dialys is.
838
Although typically considered in people with advanced
kidney disease, supportive care may be applicable to people
in earlier CKD stages. Whereas comprehensive conservative
management is usually referred to as active medical
management in people with kidney failure who choo se not
to have KRT. There are 3 distinct groups of people with
kidney failure who receive comprehensive conservative care
because provision of supportive care differs for each.
915
Descriptions of each group are shown in Table 43.
There is increasing recognition that provision of organized
care to those who are dying or choose to not pursue KRT is of
value to people with CKD and their families. Healthcare
providers involved in car ing for these people should be aler-
ted to this need.
Comprehensive conservative care is an alternative treat-
ment to KRT. This is planned, holistic, person-centered care
that includes the full integration of comprehensive conser-
vative care includ ing the following:
Detailed communication including estimating prognosis
and advance care planning
Shared decision-making
Active symptom assessment and management
Psychological, social, family, cultural, and spiritual support
Interventions to delay progression and minimize risks
of adverse events or complications, but not including
dialysis.
Evaluating the prognosis of each person with CKD is very
important because each person has a different disease pro-
gression pattern. Patient prognosis is the key information for
shared decision-making in CKD G5, which requires unbiased
information on survival and person-centered outcomes
known to matter to people with CKD: QoL, symptom
burden, and support from family and healthcare providers.
Shared decision-making helps healthcare providers, people
with CKD, and family members to reach agreement on the
treatment direction that is appropriate with the person’s
values and preferences and family goals. This process should
be performed in a culturally appropriate way with consider-
ation of appropriate health literacy.
As CK D progresses, the person with CKD will exper i-
ence more symptoms and complications related to CKD.
Therefore, active sy mptom assessment and management
are the key compone nts of com prehen sive con servative
care in CKD G5. Assessing a person’ssymptomsona
regular basis helps redire ct management toward a person’s
values and pref erences and family goals. There is limited
evidence for selecting treatment strategies due to the
complexity of CKD and differences in people and the
considerable variation in the management strategies for
different symptoms. Intervention to delay progression of
CKD is still an important component of comprehensive
conservat ive care in both CK D-related aspects (maintain
residual kidney function and reduce cardiovascular
morbidity) and psychospiritual aspects (the person and
their family members do not feel that active CKD treat-
ment is discontinued).
Advanced care planning (ACP) is a process under the
comprehensive conservative care umbrella that involves un-
derstanding, communication, and discussion between a per-
son with CKD, the family, caregiver, and healthcare providers
for the purpose of clarifying preferences for end-of-life care.
End-of-life care is the treatment during the phase where death
is inev itable. It focuses on QoL not quantity of lifetime.
Functional and cognitive decline that may happen along with
CKD progression results in difficult end-of-life conversations
involving people with CKD, families, and healthcare pro-
viders. Therefore, an integrated approach to timely ACP and
Table 43 | People with kidney failure who receive comprehensive conservative care
Category Description
Receiving conservative care Conservative care that is chosen or medically advised.
Choice-restricted conservative care Conservative care for person in whom resource constraints prevent or limit access to KRT; therefore, a choice
for conservative care cannot be recognized.
Unrecognized CKD G5 CKD is present but has not been recognized or diagnosed; therefore, a choice for conservative care
cannot be recognized.
CKD, chronic kidney disease; KRT, kidney replacement therapy.
chapter 5 www.kidney-international.org
S268
Kidney International (2024) 105 (Suppl 4S), S117–S314

palliative care spanning the continuum of CKD care is
needed. End-of-life care is underused in the management of
people with CKD G5 due to inadequate education during
nephrology training leading to poor end-of-life care discus-
sions with the person. The overall concept of supportive care,
comprehensive conservative care, and end-of-life care is
shown in Figure 56.
In different societi es or cultural areas, the form and
structure of this care may vary tremendously, and families
or religious organizations may be able to deliver suitable
and sensitive care. The details here are l isted not to be
prescriptive but rather to articulate the best practices in
communities where resources may be available and to serve
as a construct to review in those locations where resources
are more limited.
ACP is thought to be a component of all comprehensive
chronic disease management. Thus, ACP discussions are not
restricted only to those choosing supportive care.
For research recommendations, please see Chapter
6: Research recommendations.
Comprehensive conservative care
Interventions to delay
progression and minimize
complications of CKD
Psychological, social,
family, cultural, and
spiritual support
Advanced stage CKD gniyDgnitaroiretedyllacinilC
Shared decision-making and
advance care planning
Obtain consensus and ensure
consistency among all caregivers
Truthful, accurate, and comprehensive
disclosure of the prognosis to family
Physician's objective and subjective assessment
of the dying process/medical futility
Implementing the process of withholding
or withdrawing life support
to patient and appropriate support to the family
End-of-life care
Deprescribing
and reduce
monitoring
Active symptom
assessment and
management
Supportive care
Supportive care
Supportive care
Estimating prognosis
Detailed communication
Shared decision-making
Figure 56 | Relationship between supportive care, comprehensive conservative care, and end-of-life care. Kidney supportive care
emphasizes efforts to optimize quality of life and relieve suffering in people with kidney disease. This emphasis should be present for people
undergoing comprehensive conservative care, as well as those who transition to end-of-life care. CKD, chronic kidney disease
www.kidney-international.org chapter 5
Kidney International (2024) 105 (Suppl 4S), S117–S314 S269

Chapter 6: Research recommendations
The last 30 years have seen an exponential growth in the
literature relating to kidney disease. However, despite this
inclusion of people with CKD in prospective RCTs, those with
lower GFR remain under-represented. Before the KDIGO
2012 CKD guideline, a low eGFR was an exclusion criterion
for almost every large cardiovascular and BP trial. As a result,
we had a largely opinion-based literature. That is changing,
and although still low, the proportion of the total CKD
literature that is either an RCT, met a-analysis, or systematic
review has doubled from 3.3% to 6.5% of published articles
in the 5-year period ending December 2022.
Several large in ternational inter ventional studies have
been completed in the last 8 years, either specifically tar-
geting people with CKD and other comorbidities (most
notably diabetes o r CVD), or with CKD defined by eGFR
and ACR cr iter ia (e.g., Canag liflozin and Renal Endpoints in
Diabetes with Established Nephropathy Clinica l Evaluation
[CREDENCE],
514
DAPA-CKD,
513
EMPA-KIDNEY,
403
FIGARO-DKD,
534
and FIDELIO-DKD
533
). Interventional
studies targeting specific pathway s and diseas es are
increasing as well, though limited in scale due to rar it y of
conditions (e.g., IgAN, membranous nephropathy, and
systemic lupus ery thematosus, etc.).
There remain gaps in the evidence base to inform best
diagnostic testing strategies, decision-making, and processes
of care. In addition, some therapies offering promise have not
yet been adequately tested in people defined by specific
criteria with CKD (i.e., people without diabetes, children,
women, PKD, frail, elderly, etc.) and people from low-income
and low-middle-income countries. We, therefore, begin this
section detailing research recommendations with some gen-
eral guiding principles for those desig ning studies to consider
when addressing key questions that impact people living with
or at risk for CKD.
Guiding principles for research
1. To ensure that the evidence base is directly applicable to
all people with CKD, future studies should avoid auto-
matic exclusion of older people, children, and young
adults <18 years old, and give consideration to pregnant
and lactating peop le. The need for contraceptive re-
quirements for trial participation should also be reviewed.
2. Decreased GFR should not be a reason for automatic
exclusion from research studies.
3. Estimating equations equally appli cable to those with and
without CKD are required for children and adolescents.
4. For new equations predicting CVD and m ortalit y
risk as well as CKD progression, development and
implementation studies including validation in different
populations (geographic and demographic) are
required.
5. Benefit-risk ratio assessments of old, new, and future
medications should be performed by levels of GFR and
ACR and/or using validated risk equations.
6. Drug studies evaluating pharmacodynamics should
consider using validated eGFR equations or mGFR for
highly toxic drugs with a narrow therapeutic window,
especially for those frequently used in CKD. As this may
be prohibitively expensive for some medicatio ns, epide-
miologic studies may provide information for revisions to
labels for some drugs.
7. Pharmacokinetic studies in people with CKD should not
automatically exclude GFR categories G4 and G5.
8. Studies should consider me asurement of ACR in all co-
horts, whether specifically focused on CKD or not, given
additional prognostic value of this parameter for so many
outcomes.
9. All studies should ensure attention to etiology of CKD,
sex, gender, age, and SES considerations in design and
analysis of results.
10. Use of novel study designs (platform, registry-embedded,
and pragmatic trials), use of large administrative datasets,
and implementation science methodologies (e.g., causal
inference techniques) should be considered to enable the
assessment and evaluatio n of interventions, processes, and
models of care.
11. People living with CKD should be involved in clinical
studies throughout the research process from identifica-
tion of knowledge gaps to knowledge mobilization and
study design.
The following set of more specific research recommenda-
tions are organized according to chapter and are not
exhaustive. They are generated in part from identifying gaps
in knowledge during the evidence review and in part from
clinical practice and patient perspectives.
Screening
Determine whether efforts to systematically detect and treat
CKD in targeted populations in the community setting will
reduce the incidence of CVD and CKD progression to
kidney failure through earlier intervention of disease-
modifying strategies.
chapter 6 www.kidney-international.org
S270
Kidney International (2024) 105 (Suppl 4S), S117–S314

Chapter 1. Evaluation of CKD: Improving the accuracy
and sophistication of evaluation of kidney fu nctions is
critical to advancing the science a nd care of people with
CKD.
Imag ing techniques
B Determine which imaging techniques, or combi-
nation of imaging techniques, may be used to
assess kidney damage and evaluation of specific
causes of CKD.
B Develop more sophisticated imaging methods for
assessment and follow-up to aid in noninvasive
evaluation of kidney functions (GFR, tubular, and
plasma clearance).
Genetic testing
B Determine the additional value of genetic testing in
people with CKD, both with and without kidney
biopsy, for determination of cause, prognosis, and
treatment choices.
Kidney biop sy
B Determine the prevalence of kidney biopsy–related
complications in different cli nical circumstances
(age, size of kidneys, acute vs. chronic, and comor-
bidities) to inform risk estimates appropriate to
those in the current era dependent on methods (e.g.,
blind, ultrasound-guided, CT-guided, open, and
transjugular) used to obtain kidney tissue.
Novel urinary biomarkers
B Determine which novel urinary biomarkers, or com-
bination of biomarkers, aid the identification of CKD
cause (e.g., interleukin [IL]-18, kidney injury mole-
cule [KIM]-1, neutrophil gelatinase-associated lip-
ocalin [NGAL], monocyte chemoattractant pr otein
[MCP]-1, tissue inhibitor matrix metalloproteinase
[TIMP]-1, alpha-1-microglobulin, uromodulin,
epidermal growth factor [EGF], and YKL-40).
B Determine how urine cytology may aid the iden-
tification of CKD cause in specific circumstances.
Develop clinically robust and accessible tests for kidne y
functional reserve
B Determine how kidney functional reserve varies by
demographic characteristics including birthweight,
in people with and without CKD of different eti-
ologies, in kidney donors, and at different levels of
GFR and ACR.
B Evaluate sex (e.g., female, male, or intersex) dif-
ferences in GFR and kidney function al reserve at
various hormonal stages such as puberty, men-
strual cycle, pregnancy, menopause, and gender
differences (e.g., identity, roles, or relations) in
people across the life cycle.
B Evaluate changes in kidney functional reserve after AKI
episodes irrespective of baseline GFR and recovery .
Develop better tests for tubular function.
Measurement of GFR
B Harmonize and standardize existing mGFR protocols
and determine their accuracy and comparability.
B Determine whether more efficient methods for GFR
measurement (POC, shorter protocols, and subcu-
taneous administration) have adequate accuracy
and precision to be considered a “gold standard.”
B Identify barriers to the performance and imple-
mentation of mGFR in the nephrology diagnostic
repertoire.
Estimations of GFR
B Assess the diagnostic accuracy and utility of GFR
estimates using endogenous filtration markers such
as SCr and cystatin C in children and young adults
and in frail, acute, or chronically ill populations;
obese and pregnant populations; transgender,
gender-diverse, and nonbinary populations; and
transplant recipients.
B When reporting performance of eGFR in research
studies, future studies should report P
15
in addi-
tion to P
30
, with expectation that improved equa-
tions may achieve levels of accuracy approaching
that of mGFR.
B Assess the non-GFR determinants of endogenous
filtration markers such as cystatin C.
B Assess the utility of changes in eGFRcr versus
eGFRcys versus eGFRcr-cys over time for clinical
decision-making, enrollment into clinical trials,
and so on.
B Examine the effect of sex hormone status (e.g., pu-
berty, gonadectomy, or menopause), exogenous
hormone use (e.g., contraception, assisted repro-
ductive technologies, menopausal hormone therapy,
testosterone replacement therapy, or gender-affirm-
ing hormone therapy), or sex hormone deprivation
therapy (e.g., antiandrogen or antiestrogen therapy)
on serum levels of creatinine and cystatin C and their
corresponding GFR estimates and mGFR.
B Evaluate the accuracy of the CKiD U25 2021 eGFRcr
and EKFC equations in diverse cohorts outside of
North America and Europe in children younger than
5 years, in children and adolescents with obesity, and
in those with eGFR <30 ml/min per 1.73 m
2
.
Determine validity of different estimating equations for
GFR in children at different points in time (2–5 years, 5–10
years, 10–14 years, and >14 years).
B Evaluate which estim ating equations should be
used for eGFR in young adults and what criteria
should be used to transition to adult eGFR equa-
tions if not using EKFC equations.
Evaluate the utility of total urine protein loss or PCR in
comparison with ACR in the evaluation of specific kidney
diseases in both children and adults.
Evaluate the role of detection and measurement of specific
tubular proteins to identify and quantify kidney damage
across the age, sex, and gender spectrum.
Evaluate the clinical utility and diagnostic accuracy of
cystatin C/eGFR in POCT devices using standardized
criteria.
www.kidney-international.org chapter 6
Kidney International (2024) 105 (Suppl 4S), S117–S314 S271

Evaluate the cost-effectiveness and cost-utility of POCT for
creatinine and urine albumin in specific situations (rural,
remote, high r isk, and children).
Define different clinical settings and specific circumstances
in which POCT would be valuable to patients, clinicians,
and/or researchers.
Chapter 2. Risk assessment in people with CKD:
Improving the accuracy of risk assessment and demon-
strating utility and usefulness of validated risk assessment
tools in clinical practice are critical to uptake.
Determine whether persons who have a >30% decline in
eGFR while using RASi, SGLT2i, and MRAs have better out-
comes if they continue versus discontinue these medications.
Determine the clinical importance/meaning of divergence of
eGFRcys and eGFRcr in clinical practice, and whether the
divergence magnitude and/or directionvaries by demography.
Evaluate the clinical and cost utilities of equations guiding
clinical decision-making in people with CKD including
children and young people, for individual people, clini-
cians, and the healthcare system.
Develop implementation strategies and evaluation frame-
works to enable assessment of the potential barriers and
facilitators of validated equations fo r CKD populations,
including the new equations for CVD and mortality out-
comes as well as for proximal CKD progression.
Risk scores derived from validated equations should be tested
as both inclusion criter ia for enrichment of study cohorts as
well as potential surrogate endpoints in clinical tr ials.
Evaluate the difference in performance characteristics of
validated risk equations for eGFR using endogenous filtration
markers such as cystatin C, creatinine, or both (eGFRcys,
eGFRcr, and eGFRcr-cys) for kidney failure, cardiovascular
events, and all-cause mortality, and pregnancy and fetal out-
comes in a variety of populations (i.e., age, sex, and region).
Chapter 3. Delaying CKD progression and managing its
complications: There is a paucity of well-designed studies
evaluating combination therapies and nutritional strategies
in different populations with CKD and evaluating specific
target values for interventions in laboratory abnormalities,
which generates uncertainty and confusion for both clini-
cians and patients.
Generate more evidence on the effect of nutritional thera-
pies (e.g., varying levels of protein restr iction with and
without supplementation [e.g., ketoanalogs]) documenting
benefits (e.g., delaying progression) versus potential harms
(e.g., patient intolerance and malnutrition).
Evaluate different nutrition regimens in larger, longer-term
RCTs than those performed to date using pragmatic designs
to enable generalizability.
Evaluate the effects of plant-based protein diets and diets such
as the Mediterranean, Okinawan, and DASH diets versus
animal-based protein diets on risk of CKD progression,
metabolic acidosis, hy perphosphatemia, and hyperkalemia.
Evaluate the benefit-risk ratio and impact on QoL of dietary
restriction (i.e., protein restriction vs. no protein restr ic-
tion) in people with CKD receiving optimal medical ther-
apy (e.g., ACEi/SGLT2i/ns-MRA).
B Does this vary by age, sex, ACR, initial eGFR, and
etiology of CKD?
Evaluate the role of sodium restriction in combination with
optimal medical therapy in prevention of progression of
CKD in people with CKD, including a range of baseline
BPs, ages, sex, and etiology of CKD.
Examine the safety and efficacy of SGLT2i in the CKD
population subgroups understudied in completed large
RCTs (e.g., people with PKD and T1D, children, young or
older adults, transgender, gender-diverse and nonbinary
people, and women at different ages/hormonal status
including pregnancy and lactation).
Evaluate the cost-effectiveness of strategies to prevent the
progression of CKD in people with relatively low (e.g.,
<5%) risk of kidney failure within 5 years, by etiology, age,
sex, and gender.
Evaluate and determine if additional clinically available
biomarkers predict outcomes in people with CKD without
diabetes and with lower ACR <30 mg/g (<3 mg/mmol).
Determine the safety and efficacy of SGLT2i for prevention of
progression of CKD in children and young adults with CKD.
Do inhibitors of the aldosterone pathway have a role in
prevention of progression of CKD and cardiovascular
outcomes in people with CKD, including those with an
ACR <30 mg/g (<3 mg/mmol), with and without T2D?
What are the net benefit-risks particularly at higher baseline
serum potassium (e.g., K
þ
>5.0 mmol/l), and are effects
modified by concurrent use of an SGLT2i?
B Including young adults, women with different hor-
monal status/supplementation, and varying etiology.
Evaluate the safety and efficacy of introducing therapy w ith
an SGLT2i and an inhibitor of the aldosterone pathway
simultaneously as compared with sequentially in people
with CKD.
Evaluate the effects of GLP-1 RA on risk of adverse car-
diovascular outcomes and kidney disease progression in
people with various etiologies of CKD. Trials should includ e
people without diabetes, particularly if they are overweight
or obese.
Evaluate the impact of correction of metabolic acidosis, at
different levels of serum bicarbonate with respect to benefits
in terms of CKD progression, muscle wasting, development,
or exacerbation of bone disease, protein malnutrition,
growth (in children and adolescents), and mortality.
Evaluate the efficacy and safety of dietary interventions in
specific groups of people with CKD (e.g., diabetes vs. no
diabetes). Outcomes should include PROMs and clinically
important cardiovascular and kidney outcomes, as well as
serum potassium concentration.
Evaluate the impact on kidney, cardiovascular, and safety
outcomes by maintaining and optimizing RAASi despite
chapter 6 www.kidney-international.org
S272
Kidney International (2024) 105 (Suppl 4S), S117–S314

hyperkalemia in people with CKD stratified by grade of
heart failure, ACR, etiology of CKD, age, and sex.
Evaluate the impact on patient outcomes and resource
utilization of different strategies to address hyperkalemia
identified in outpatient populations with CKD.
Investigate best strategies to prevent hyperkalemia using
resource utilization outcomes such as reduction of hospi-
talizations, emergency department presentations, and
additional investigations.
Evaluate the impact of low-potassium diets on serum po-
tassium, mortality, and QoL in patients with CKD, by age,
sex, and etiology.
Large RCTs are needed to address effects of the use of po-
tassium exchange agents on clinical outcomes, such as
laboratory or hospital visits, cardiovascular outcomes, and
CKD progression.
Evaluate the value of uric acid–lowering therapies on CKD
and CVD outcomes in populations at risk of either or both,
ensuring representation of a range of ages, sex, and
ethnicities.
What dietary modification reduces serum uric acid and risk
of gout in people with CKD?
What are the safety and efficacy of different symptomatic
treatment strategies for acute gout in people with CKD
(including a short course of NSAIDs as a potential
comparator)?
RCTs are needed to assess the efficacy and safety of long-
term, low-dose colchicine on risk of CVD, and gout in CKD.
Evaluate the clinical and cost-effectiveness of PCSK-9 in-
hibitors in people with CKD (vs. statins), by age, sex, and
etiology.
Assess effects of antiplatelet agents, such as low-dose
aspirin, for primary prevention of CVD in people with
CKD in large RCTs, stratified by age, sex, risk of event, and
ethnicity.
Develop and refine CKD-specific risk assessment tools for
CVD and major bleeding, so as to provide more individ-
ualized decision-making for the use of all agents (including
deprescribing).
Identify which people with CKD may particularly benefit
from invasive management of ischemic heart disease versus
maximal medical therapy in people with CKD stratified by
age, sex, frailty, and etiology of CKD.
New thromboprophylaxis risk scores incorporating CKD-
specific predictors or ensemble modeling to combine
existing risk scores to improve risk prediction in people
with CKD are needed.
Chapter 4. Medication management and drug steward-
ship in CKD: Appropriate dosing of medications according
to different biological parameters in people with CKD is
critical to evaluating benefits and risks; thus, studies tar-
geted at answering these questions will be valuable.
Evaluate the effects of age, sex, body size, and etiology on
pharmacodynamics and pharmacokinetics of specific drugs
in people with CKD.
Assess the non-GFR determinants of cystatin C and how
serum cystatin C concentration may be influenced by
medications.
Evaluate the role of kinetic eGFR to inform and improve drug
dosing and administration in people in a nonsteady state.
Evaluate the utility of endogenous filtration markers such as
cystatin C (eGFRcys) to inform drug dosing and
administration.
Identify settings in which the use of eGFRcys or eGFRcr-cys
can improve the safety and effectiveness of specific medi-
cations relative to eGFRcr.
Evaluate different strategies (i.e., consumer engagement,
generic vs. specific reminders, etc.) in people with CKD of
different ages, sex, gender, and etiology to ascertain the
impact on compliance and adherence.
Evaluate the impact of electronic clinical decision support
systems to improve the medication management of people
with CKD.
Evaluate the impact of deprescribing of nonessential/
nonevidence-based medications on patient adherence and
outcomes.
Evaluate the impact of newer agents (e.g., SGLT2i and ns-
MRAs) in patients intolerant of ACEi/ARB.
Chapter 5. Optimal models of care: The key components
of care models for different conditions have not been well-
identified, but are known to be modified by age, sex, gender,
and etiology. Implementation of known effective treatments
lags behind the evidence, and use of implementation science
techniques is critical to ultimately enable clinicians and
patients to benefit from advances in care model development
and interventional studies.
Evaluate the utility and barriers to using validated tools in
clinical practice to assess the specific symptoms or out-
comes of importance to peop le with CKD of different ages,
sex, gender, and ethnicity/region.
Develop and evaluate/validate clinically relevant and reli-
able tools for health literacy and workability for use in
different populations (i.e., age, gender, and region).
Evaluate the burden, yield, variabili ty, and stability of
routine screening for a wide range of common symptoms in
people with CKD G3–G5, irrespective of age, sex, gender,
and etiology of CKD.
Quantitative and qualitative methods should be developed by
which identification and classification of specific common
symptoms experienced by people with CKD are captured.
Platform studies to evaluate the value of different in-
terventions for common symptoms should be developed,
enabling assessment of established and new therapies in a
rigorous manner.
Using implementation science methods, evaluate best
methods to ensure uptake of proven therapies for symptom
management into clinical care.
Determine the components essential for transition clinics to
have a positive impact on the outcomes of young people
with CKD, including cost-effectiveness and patient-re-
ported outcomes.
www.kidney-international.org chapter 6
Kidney International (2024) 105 (Suppl 4S), S117–S314 S273

Methods for guideline development
Aim
The aim of this project was to update the KDIGO 2012
Clinical Practice Guideline for the Evaluation and Manage-
ment of Chronic Kidney Disease.
1
The guideline development
methods are described below.
Overview of the process
This guideline adhered to international best practices for
guideline development (Appendix B: Supplementary Table
S2)
916,917
and have been reported in accordance with the
AGREE II reporting checklist.
918
The processes undertaken
for the development of the KDIGO 2024 Clinical Practice
Guideline for the Evaluation and Management of CKD are
described below.
Appointing Work Group members and the ERT
Finalizing guideline development methodology
Defining scope of the guideline
Developing and registerin g protocols for systematic reviews
Implementing literature search strategies to identify the
evidence base for the guideline
Selecting studies according to predefined inclusion criteria
Conducting data extraction and risk of bias assessment of
included studies
Conducting evidence syntheses, including meta-analysis
where appropriate
Assessing the certainty of the evidence for each critical
outcome
Finalizing guideline recommendations and supporting
rationale
Grading the strength of the recommendations based on the
overall certainty of the evidence and other considerations
Convening a public review of the guideline draft in July
2023
Updating systematic reviews
Amending the guideline based on the external review
feedback and updated systematic revie ws
Finalizing and publishing the guideline.
Commissioning of Work Group and ERT. KDIGO and the
Co-Chairs assembled and engaged a Work Group w ith
expertise in pediatr ic, adult, and geriatric nephrology,
including both dialysis and transplant specialists; primary
care; internal medicine; dietetics; nursing; women’s health;
clinical trials; epidemiology; medical decision-making; and
public health; as well as people living with CKD. Johns
Hopkins University, with expertise in nephrolog y, evidence
synthesis, and guideline development, was contracted as the
ERT and was tasked with conducting the evidence reviews.
The ERT coordinated the methodological and analytical
processes of guideline development, including literature
searching, data extraction, risk-of-bias assessment, evidence
synthesis and meta-analysis, grading the certainty of the evi-
dence per critical outcome, and grading the overall certainty
of the evidence for the recommendations. The Work Group
was responsible for wr iting the recommendations and the
underlying rationale, grading the strength of the recom-
mendations, and developing practice points.
Defining scope and topics and formulating key clinical ques-
tions.
The KDIGO 2012 CKD guideline was reviewed by the
Co-Chairs to identify topics to be included in the 2024
guideline. Scoping reviews of these topics were conducted by
the ERT to provide an overview of the available evidence base
and to identify existing relevant systematic reviews.
The Risk of Bias in Systematic Reviews (ROBIS) tool was
used to assess the risk of bias of the existing reviews. When
high-quality systematic reviews were identified during the
scoping reviews, the ERT conducted an updated search based
on the existing review and extracted information from the
newly identified studies. This information was added to the
existing review data and analyzed as appropriate.
For topics that did not map to current high-qualit y re-
views, de novo systematic reviews were undertaken. Protocols
for each review were developed by the ERT and reviewed by
the Work Group. Protocols were registered on PROSPERO
(https://www.crd.york.ac.uk/prospero/). Systematic reviews
were conducted in accordance with current standards,
including those from the Cochrane Handbook.
919
Details of the Population, Intervention, Comparator,
Outcome and Study design (PICOS) of the questions are pro-
vided in Table 44.
23,316,318,415,511,609,920–926
Information about
existing reviews that were used is included in these tables.
For some topics not predefined in the Scope of Work, the
ERT extracted the certainty of evidence from existing high-
quality systematic reviews, as available. Details of the PICOS
for these questions are also provided in Table 44.
Literature searches and article selection. Searches for RCTs
were conducted on PubMed, Embase, and the Cochrane
Central Register of Controlled Trials (CENTRAL), and
searches for diagnosis/prognosis studies were conducted on
PubMed, Embase, and CINAHL. For topics with available
existing reviews, the review was used and an updated search
was conducted. The search strategies are provided in
Appendix A: Supplementary Table S1.
To improve efficiency and accuracy in the title/abstract
screening process and to manage the process, search results
were uploaded to a web-based screening tool, PICO Portal
(www.picoportal.net). PICO Portal uses machine learning to
sort and present those citations most likely to be promoted to
full-text screening first. The titles and abstracts resulting from
methods for guideline development www.kidney-international.org
S274
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table 44 | Clinical questions and systematic review topics in PICOS format
Chapter 1 Evaluation of chronic kidney disease (CKD)
Clinical question What is the diagnostic and prognostic benefit and safety of kidney biopsy among people with CKD?
Population Adults and children with suspected or diagnosed CKD
Intervention (index test) Native kidney biopsy
Comparator For studies evaluating diagnostic or prognostic benefit, clinical or standard diagnosis, or prognosis
For studies evaluating safety, no comparator
Outcomes Critical outcomes: mortality, perirenal hematoma (perinephric hematoma), and retroperitoneal hemorrhage
Other outcomes: diagnostic and prognostic benefit, macroscopic hematuria, transfusion, need for embolization,
nephrectomy, AKI, and major complications
Study design Noncomparative studies, before and after studies
Existing systematic review
used for handsearching
Poggio ED, McClelland RL, Blank KN, et al. Systematic review and meta-analysis of native kidney biopsy complications.
Clin J Am Soc Nephrol. 2020;15:1595–602.
920
SoF tables Supplementary Table S4
Search date March 2023
Citations screened/included
studies
1582/65
Supplementary Figure S1
Clinical question What is the diagnostic accuracy of eGFR based on measurements of cystatin C, creatinine, or their combination
compared with mGFR among people with and without CKD?
Population Adults and children with or without CKD
Intervention (index test) eGFR based on measurements of cystatin C (eGFRcys), creatinine (eGFRcr), cystatin C and creatinine (eGFRcr-cys)
Comparator mGFR (using urinary or plasma clearance of the exogenous filtration marker)
Outcomes Critical outcomes: measurement bias (eGFR – mGFR), accuracy (P
30
and P
15
)
Other outcomes: probability of being classified in each eGFR category
Study design Cross-sectional
Existing systematic reviews None
SoF tables Supplementary Table S3
Search date August 2022
Citations screened/included
studies
1848/47
Supplementary Figure S2
Clinical question In children and young adults with suspected or diagnosed CKD, what is the accuracy of the albumin-to-
creatinine ratio (ACR) and protein-to-creatinine ratio (PCR) compared with 24-hour excretion of albumin or
protein?
Population Children and young adults (age <25 years) with suspected or diagnosed CKD
Intervention (index test) ACR and PCR
Comparator Albuminuria or proteinuria determined from 24-hour urine collection
Outcomes Outcomes: median (IQR) or difference between intervention and comparison, sensitivity and specificity for detection,
and diagnosis of significant proteinuria
Study design Prospective, observational studies
Existing systematic review
used for handsearching
National Institute for Health and Care Excellence (NICE). Evidence review for the accuracy of albumin:creatinine ratio vs.
protein creatinine ratio measurements to quantify proteinuria in children and young people with CKD. Chronic Kidney
Disease: Evidence Review B. NICE; 2021.
921
SoF tables No summary of findings table
Search date July 2022
Citations screened/included
studies
485/0
Supplementary Figure S3
Clinical question What is the diagnostic accuracy and reproducibility of point-of-care (POC) blood creatinine compared with
laboratory-based tests among people with suspected or diagnosed CKD?
Population Adults and children
Intervention (index test) Quantitative internationally standardized POC creatinine tests
Comparator Laboratory-based methods for measuring SCr
Outcomes Critical outcomes: measurement bias, analytical sensitivity (limit of detection), and analytical variability (coefficient of
variation)
Study design Cross-sectional
Existing systematic reviews
used for handsearching
National Institute for Health and Care Excellence. Point-of-care creatinine devices to assess kidney function before CT
imaging with intravenous contrast. NICE Guideline [NG37]. NICE; 2019.
316
Corbett M, Duarte A, Llewellyn A, et al. Point-of-care creatinine tests to assess kidney function for outpatients requiring
contrast-enhanced CT imaging: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1–248.
922
SoF tables No summary of findings table
Search date January 2023
Citations screened/included
studies
986/55
Supplementary Figure S4
Clinical question What is the diagnostic accuracy of quantitative and semiquantitative protein or albumin urine dipstick tests
compared with laboratory-based tests among people with suspected or diagnosed CKD?
Population Adults and children
(Continued on following page)
www.kidney-international.org methods for guideline development
Kidney International (2024) 105 (Suppl 4S), S117–S314 S275

Table 44 | (Continued) Clinical questions and systematic review topics in PICOS format
Chapter 1 Evaluation of chronic kidney disease (CKD)
Intervention (index test) Machine-read quantitative or semiquantitative protein or albumin urine dipstick tests
Comparator Laboratory-based methods for measuring urinary protein or albumin (e.g., 24-hour urinary sample, spot urine ACR, or
PCR)
Outcomes Critical outcomes: measurement bias, analytical sensitivity (limit of detection), analytical variability (coefficient of
variation), and analytic specificity (or numbers to calculate)
Other outcomes: probability of being classified in each albuminuria or proteinuria stage
Study design Cross-sectional
Existing systematic reviews
for handsearching
McTaggart MP, Newall RG, Hirst JA, et al. Diagnostic accuracy of point-of-care tests for detecting albuminuria: a
systematic review and meta-analysis. Ann Int Med. 2014;160:550–557.
318
SoF tables Supplementary Table S5
Search date July 2022
Citations screened/included
studies
2184/65
Supplementary Figure S5
Chapter 2 Risk assessment in people with CKD
Clinical question Are kidney failure prediction equations good predictors of progression, kidney failure, or end-stage renal
disease?
Population Adults, children, and young people with CKD G1-G5
Predictor Kidney failure risk equations (e.g., Tangri equation [Kidney Failure Risk Equation])
Outcomes Prognostic performance:
Calibration (goodness of measures, e.g., R
2
, Brier score, and Hosmer-Lemeshow test)
Discrimination (e.g., sensitivity/specificity; area under the curve [AUC] from receiver operating characteristic [ROC] and
area under the receiver operating characteristic curve [AUROC]; C-statistic)
Study design Systematic review
Existing systematic review National Institute for Health and Care Excellence. Evidence review for the best combination of measures to identify
increased risk of progression in adults, children and young people. Chronic Kidney Disease: Evidence Review F. NICE
Evidence Reviews Collection; 2021. NICE.
415
SoF tables Supplementary Tables S6–S9
Search date N/A
Chapter 3 Delaying CKD progression and managing its complications
Clinical question What is the effect of sodium-glucose cotransporter-2 inhibitors (SGLT2i) compared with placebo, usual care, or
an active comparator among people with CKD in terms of mortality, progression of CKD, complications of CKD,
and adverse events?
Population Adults and children with CKD; subgroup of people (1) with type 2 diabetes (T2D), (2) without T2D, (3) with heart failure,
and (4) without albuminuria
Intervention SGLT2i (canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, ipragliflozin, luseogliflozin, remogliflozin, sotagliflozin,
tofogliflozin)
Comparator Active comparator (e.g., another glucose-lowering agent), placebo, or usual care
Outcomes Critical outcomes: kidney failure (including CKD progression) and all-cause hospitalizations
Other outcomes: mortality, change in eGFR (including acute changes), complications of CKD, and adverse events
Study design Randomized controlled trials (RCTs)
Existing systematic review
data included
Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease. Kidney Int. 2022;102(5S):S1–S127.
23
Nuffield Department of Population Health Renal Studies Group, SGLT Inhibitor Meta-Analysis Cardio-Renal Trialists’
Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes:
collaborative meta-analysis of large placebo-controlled trials. Lancet 2022;400:1788–1801.
511
SoF tables Supplementary Table S10
Search date NDPH 2022: September 2022; KDIGO 2022: December 2021; Updated: April 2023
Citations screened/included
studies
252/2
Supplementary Figure S6
Clinical question What is the effect of mineralocorticoid receptor agonists (MRAs) compared with placebo, usual care, or an active
comparator among people with CKD but not T2D in terms of mortality, progression of CKD, complications of
CKD, and adverse events?
Population Adults and children with CKD but not diabetes
Intervention Steroidal MRAs (canrenone, eplerenone, spironolactone); nonsteroidal MRAs (esaxerenone, finerenone)
Comparator Active comparator, placebo, or usual care
Outcomes Critical outcomes: kidney failure and all-cause hospitalizations
Other outcomes: mortality, progression of CKD, complications of CKD, and adverse events
Study design RCTs
Existing systematic review
used for handsearching
Chung EY, Ruospo M, Natale P, et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for
preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020;10:Cd007004.
923
SoF tables Supplementary Table S16
(Continued on following page)
methods for guideline development www.kidney-international.org
S276
Kidney International (2024) 105 (Suppl 4S), S117–S314

Table 44 | (Continued) Clinical questions and systematic review topics in PICOS format
Chapter 3 Delaying CKD progression and managing its complications
Search date January 2020
Citations screened/included
studies
106/19
Supplementary Figure S7
Clinical question What is the effect of MRAs compared with placebo, usual care, or an active comparator among people with CKD
and T2D in terms of mortality, progression of CKD, complications of CKD, and adverse events?
Population Adults and children with CKD and diabetes and subgroup of people with heart failure
Intervention Steroidal MRAs (canrenone, eplerenone, and spironolactone) and nonsteroidal MRAs (esaxerenone and finerenone)
Comparator Active comparator, placebo, or usual care
Outcomes Critical outcomes: kidney failure and all-cause hospitalizations
Study design RCTs
Existing systematic reviews Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for
Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022 Nov;102(5S):S1–S127.
23
SoF tables No summary of findings table (see KDIGO Diabetes Guideline Data Supplement)
Search date December 2021
Citations screened/included
studies
106/44
Clinical question What is the effect of glucagon-like peptide-1 receptor agonists (GLP-1 RA) compared with placebo, usual care, or
an active comparator among people with CKD but not T2D in terms of mortality, progression of CKD,
complications of CKD, and adverse events?
Population Adults and children with CKD but not diabetes
Intervention GLP-1 RA (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide)
Comparator Active comparator (e.g., another glucose-lowering agent), placebo, or usual care
Outcomes Critical outcomes: kidney failure and all-cause hospitalizations
Study design RCTs
Existing systematic reviews
used for handsearching
Kamdar A, Sykes R, Morrow A, et al. Cardiovascular outcomes of glucose lowering therapy in chronic kidney disease
patients: a systematic review with meta-analysis. Rev Cardiovasc Med. 2021;22:1479–1490.
924
Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease. Kidney Int. 2022;102(5S):S1–S127.
23
SoF tables No summary of findings table
Search date Kamdar 2021: March 2021; KDIGO 2022: December 2021
Citations screened/included
studies
65/0
Supplementary Figure S8
Clinical question What is the effect of GLP-1 RA compared with placebo, usual care, or an active comparator among people with
CKD and T2D in terms of mortality, progression of CKD, complications of CKD, and adverse events?
Population Adults and children with CKD and diabetes; subgroup of people with heart failure
Intervention GLP-1 RA (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide)
Comparator Active comparator (e.g., another glucose-lowering agent), placebo, or usual care
Outcomes Critical outcomes: kidney failure and all-cause hospitalizations
Study design RCTs
Existing systematic reviews Kidney Disease: Improving Global Outcomes Diabetes Work Group. KDIGO 2022 Clinical Practice Guideline for Diabetes
Management in Chronic Kidney Disease. Kidney Int. 2022 Nov;102(5S):S1-S127.
23
SoF tables No summary of findings table (see KDIGO Diabetes Guideline Data Supplement)
Search date December 2021
Citations screened/included
studies
154/19
Clinical question What is the effect of uric acid–lowering therapy compared with placebo, usual care, or an active comparator
among people with CKD and hyperuricemia in terms of mortality, progression of CKD, complications of CKD,
and adverse events?
Population Adults and children with CKD and hyperuricemia with subgroups for symptomatic and asymptomatic hyperuricemia
Intervention Allopurinol, benzbromarone, febuxostat, lesinurad, oxipurinol, pegloticase, probenecid, rasburicase, sulfinpyrazone, and
topiroxostat
Comparator Active comparator (e.g., another uric acid–lowering therapy), placebo, or usual care
Outcomes Critical outcomes: kidney failure, cutaneous reactions, hypersensitivity, and hepatotoxicity
Other outcomes: all-cause mortality, cardiovascular mortality, eGFR, ACR, cardiovascular events, and gout
Study design RCTs
Existing systematic reviews
for hand-searching and
updating
Sampson AL, Singer RF, Walters GD. Uric acid lowering therapies for preventing or delaying the progression of chronic
kidney disease. Cochrane Database Sys Rev 2017;10:Cd009460.
609
SoF tables Supplementary Tables S11 and S12
Search date March 2023
Citations screened/included
studies
1859/30
Supplementary Figure S9
(Continued on following page)
www.kidney-international.org methods for guideline development
Kidney International (2024) 105 (Suppl 4S), S117–S314 S277

the searches were initially screened independently by 2
members of the ERT. One screener was used when the recall
rate of citations promoted to full-text screening reached at
least 90% and then title and abstract screening was stopped
when the recall rate of citations promoted to full-text was at
least 95%. Citations deemed potentially eligible at the title
and abstract stage were screened independently by 2 ERT
members at the full-text level. At both title/abstract and full-
text screening disagreements about eligibility were resolved by
consensus, and, as necessary through discussion among the
ERT members.
Search dates, number of citations that were screened, and
number of eligible studies are included in Table 44.
Supplementary Figures S1–S12 include PRISMA diagrams
for each systematic review.
A total of 30,861 citations were screened. Of these, 145
RCTs and 232 nonrandomiz ed studi es were included in the
evidence review (Figure 57).
Data extraction. Data extraction, from studies and existing
systematic reviews, was performed by a member of the ERT
and confirmed by a second member of the ERT. Any differ-
ences among members of the ERT were resolved through
discussion. A third reviewer was included if consensus could
not be achieved.
Risk of bias of studies and systematic reviews. The majority
of reviews undertaken were intervention reviews that
Table 44 | (Continued) Clinical questions and systematic review topics in PICOS format
Chapter 3 Delaying CKD progression and managing its complications
Clinical question What is the effect of aspirin compared with placebo in terms of the primary prevention of cardiovascular
disease (CVD) and safety among people with CKD?
Population Adults and children with CKD at risk for CVD (i.e., people must not have established CVD)
Intervention Aspirin
Comparator Placebo
Outcomes Critical outcomes: incident CVD events, bleeding (intracranial hemorrhage, major extracranial hemorrhage, and
clinically relevant nonmajor bleeding)
Study design RCTs
Existing systematic reviews
updated
Pallikadavath S, Ashton L, Brunskill NJ, et al. Aspirin for the primary prevention of cardiovascular disease in individuals
with chronic kidney disease: a systematic review and meta-analysis. Eur J Prev Cardiol. 2022;28:1953–1960.
925
SoF tables Supplementary Table S17
Search date August 2022
Citations screened/included
studies
2293/5
Supplementary Figure S10
Clinical question What are the effects of angiography or coronary revascularization compared with medical treatment among
people with CKD and ischemic heart disease in terms of mortality, CVD events, kidney failure, and acute kidney
injury (AKI)?
Population Adults and children with CKD and ischemic heart disease
Intervention Angiography or coronary revascularization
Comparator Medical treatment
Outcomes Critical outcomes: all-cause mortality, CVD mortality, CVD events (including composite cardiovascular events,
myocardial infarction, and heart failure), kidney failure, and AKI
Other outcomes: patient-reported outcomes
Study design RCTs
Existing systematic reviews None
SoF tables Supplementary Table S13
Search date March 2023
Citations screened/included
studies
3521/5
Supplementary Figure S11
Clinical question What are the effects of non–vitamin K antagonist oral anticoagulants (NOACs) (also known as direct-acting oral
anticoagulants [DOACs]) with or without warfarin compared with placebo or warfarin alone among people with
CKD and atrial fibrillation in terms of stroke and bleeding risks?
Population Adults and children with CKD and atrial fibrillation
Intervention NOAC/DOAC (dabigatran, apixaban, edoxaban, rivaroxaban) with warfarin and NOAC/DOAC alone
Comparator Warfarin, placebo
Outcomes Critical outcomes: stroke (including TIA), bleeding (including intracranial hemorrhage, major bleeding, and clinically
relevant nonmajor bleeding)
Study design RCTs
Existing systematic reviews
updated
Kimachi M, Furukawa TA, Kimachi K, et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic
embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev.
2017;11:CD011373.
926
SoF tables Supplementary Tables S14 and S15
Search date March 2023
Citations screened/included
studies
3340/7
Supplementary Figure S12
ACR, albumin- to-creatinine ratio; AKI, acute kidney injury; IQR, intraquartile range; N/A, not applicable; PICOS, Population, Intervention, Comparator, Outcomes, Study design;
RCT, randomized controlled trial; SCr, serum creatinine; SoF, summary of findings; TIA, transient ischemic attack.
methods for guideline development www.kidney-international.org
S278
Kidney International (2024) 105 (Suppl 4S), S117–S314

included RCTs. For these reviews, the Cochrane Risk of Bias 2
tool was used to assess risk of bias for RCTs based on the
randomization process, deviations from the intended in-
terventions, missing outcome data, measurement of the
outcome, and selection of the reported results.
927
The Quality Assessment of Diagnostic Accuracy Studies
(QUADAS-2) tool was used to assess study limitations of
diagnostic studies based on the following items
928
:
Could the selection of patients have introduced bias (pa-
tient selection)?
Could the conduct or interpretation of the index test have
introduced bias (index test)?
Could the reference standard, its conduct, or its inter pre-
tation have introduced bias (reference standard)?
Could the patient flow have introduced bias (flow and timing)?
Applicability
Are there concerns that the included patients and
setting do not match the review question?
Are there concerns that the index test, its conduct,
or interpretation differ from the review question?
Are there concerns that the target condition as
defined by the reference standard does not match
the question?
The ROBIS tool was used to assess risk of bias in sys-
tematic reviews based on study eligibility criteria, identifica-
tion and selection of studies, data collection and study
appraisal, and overall risk of bias.
929
All risk-of-bias assessments were conducted independently
by 2 members of the ERT, with disag reements resolved by
internal discussion and consultation with a third ERT mem-
ber, as needed.
Evidence synthesis and meta-analysis. Measures of treatment
effect.
For dichotomous outcomes, a pooled effect estimate
was calculated as the RR between the trial arms of RCTs, with
each study weighted by the inverse variance, using a random-
effects model with the DerSimonian and Laird formula for
calculating between-study variance.
930
For continuous
outcomes, a standardized mean difference was calculated by
using a random-effects model with the DerSimonian and
Laird formula.
930
Data synthesis. Meta-analysis was conducted if there were 2
or more studies that were sufficiently similar with respect to
key variables (population characteristics, study duration, and
comparisons).
We combined studies of interventions in the same class
when reporting outcomes. If there was substantial heteroge-
neity (I
2
>50%) in pooled estimates for any outcome, we
stratified by the type of intervention before conducting the
pooled analyses.
Pooled sensitivity and specificity was calculated using a
random-effects model in studies addressing biopsy diagnosis
and prognosis using the Freeman-Tukey double arcsine
transformation to calculate the pooled estimate.
931
The
binomial exact method to calculate the CIs was used.
932
Included RCTs:
• SGLT2: 79 RCTs (278 reports)
• MRA (without type 2 diabetes): 19 RCTs (33 reports)
• GLP-1 (without type 2 diabetes): 0 RCTs
• Uric acid: 30 studies (32 reports)
• Aspirin: 5 RCTs
• Angiography: 5 RCTs (7 reports)
• NOAC: 7 RCTs (13 reports)
Included nonrandomized studies:
• Biopsy: 65 studies*
• eGFR: 47 studies (48 reports)
• ACR_PCR: 0 studies
• POC creatinine: 55 studies
• POC dipstick: 65 studies (66 reports)
Included studies:
RCTs: 145 (368 reports)
Non-randomized: 232 studies (234 reports)
* 38 studies included in the analyses
Nonrandomized studies (cross-sectional, pre-post,
prospective observational, noncomparative):
• PubMed: 4944
• Embase: 5196
• CINAHL: 146
• Central: 82
• Other reviews: 68
• Handsearching: 74
Randomized controlled trials identied from databases:
• PubMed: 7030
• Embase: 5716
• Cochrane Central: 6999
• KDIGO Diabetes 2022 GL: 393
• Other reviews: 153
• Handsearching: 60
Figure 57 | Search yield and study flow diagram. ACR, albumin-to-creatinine ratio; CINALL, Cumulative Index to Nursing and Allied Health
Literature; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; KDIGO, Kidney Disease: Improving Global Outcomes; MRA,
mineralocorticoid antagonists; NOAC, non–vitamin K antagonist oral anticoagulant; PCR, protein-to-creatinine ratio; POC, point of care; RCT,
randomized controlled trial; SGLT2, sodium-glucose cotransporter-2.
www.kidney-international.org methods for guideline development
Kidney International (2024) 105 (Suppl 4S), S117–S314 S279

Assessment of heterogeneity. Heterogeneity among the trials
for each outcome was tested using a standard
c
2
test using a
significance level of
a
#0.10. Heterogeneity was also assessed
with an I
2
statistic, which describes the variability in effect
estimates that is due to heterogeneity rather than random
chance. A value greater than 50% was considered to indicate
substantial heterogeneity.
933
Grading the certainty of the evidence and the strength of a
guideline recommendation.
The certainty of evidence for each
critical outcome was assessed by the ERT using the GRADE
approach.
934,935
For outcomes based on data from RCTs, the
initial grade for the certainty of the evidence is considered to
be high. The certainty of the evidence is lowered in the event
of study limitations; important inconsistencies in results
across studies; indirectness of the results, including
uncertainty about the population, intervention, outcomes
measured in trials, and their applicability to the clinical
question of interest; imprecision in the evidence review
results; and concerns about publication bias. For
imprecision, data were benchmarked against optimal
information size,
936
low event rates in either arm, CIs that
indicate appreciable benefit and harm (25% decrease and
25% increase in the outcome of interest), and sparse data
(only 1 study), all indicating concerns about the precision
of the results.
936
The fi nal grade for the certainty of the
evidence for an outcome could be high (A), moderate (B),
low (C), or ver y low (D) (Tables 45 and 46).
Summary of findings (SoF) tables. SoF tables were developed
using GRADEpro (https://www.gradepro.org/). The SoF ta-
bles include a description of the population, intervention, and
comparator and, where applicable, the results from the data
synthesis as relative and absolute effect estimates. The grading
of the certainty of the evidence for each critical outcome is
also provided in these tables. The SoF tables are available in
Appendix C and Appendix D of the Data Supplement
published alongside the guideline or at https://kdigo.org/
guidelines/ckd-evaluation-and-management/.
Updating and developing the guideline state-
ments.
Recommendations from the KDIGO 2012 Clinical
Practice Guideline for the Evaluation and Management of
Chronic Kidney Disease were considered in the context of
new evidence by the Work Group Co-Chairs and Work Group
members, and updated as appropriate.
1
Practice points were
not yet proposed as a sep arate category in 2012, so the
KDIGO 2024 Work Group considered the following
options: where new evidence did not suggest a change to
graded recommendations, the statements were retained as
graded recommendations; graded recommendations were
updated where appropriate based on new evidence; existing
recommendations that fit the criter ia for practice points
were rewritten as practice points, and new guideline
statements (both recommendations and practice points)
were generated for new clinical questions from the 2024
update.
Table 45 | Classification for certainty of evidence
Grade Certainty of evidence Meaning
A High We are confident that the true effect is close to the estimate of the effect.
B Moderate The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
C Low The true effect may be substantially different from the estimate of the effect.
D Very low The estimate of effect is very uncertain, and often, it will be far from the true effect.
Table 46 | GRADE system for grading the certainty of evidence
Study design
Step 1—starting grade of the
certainty of the evidence Step 2—lower grade Step 3—raise grade for observational studies
RCTs High Study limitations:
–1 serious
–2 very serious
Strength of association
þ1 large effect size (e.g., <0.5 or >2)
þ2 very large effect size (e.g., <0.2 or >5)
Moderate Inconsistency:
–1 serious
–2 very serious
Evidence of a dose-response gradient
Observational studies Low Indirectness:
–1 serious
–2 very serious
All plausible confounding would reduce the demonstrated effect
Very low Imprecision:
–1 serious
–2 very serious
Publication bias:
–1 serious
–2 very serious
GRADE, Grading of Recommendations Assessment, Development, and Evaluation; RCT, randomized controlled trial.
methods for guideline development www.kidney-international.org
S280
Kidney International (2024) 105 (Suppl 4S), S117–S314

Grading the strength of the recommendations. The strength
of a recommendation was graded by the Work Group as Level
1orLevel2(Table 47). The strength of a recommendation was
determined by the balance of benefits and harms across all
critical and important outcomes, the grading of the overall
certainty of the evidence, patient values and preferences,
resource use and costs, and other considerations (Table 48).
Balance of benefits and harms. The Work Group determined
the anticipated net health benefit on the basis of expected
benefits and harms across all critical outcomes from the
underlying evidence review.
The overall certainty of the evidence. The overall certai nty of
the evidence for each recommendation is determined by the
certainty of evidence for critical outcomes. In general, the
overall certainty of evidence is dictated by the critical
outcome with the lowest certainty of evidence.
936
This could
be modified based on the relative importance of each
outcome to the populati on of interest. The overall certainty
of the ev idence was graded high (A), moderate (B), low
(C), or very low (D) (Table 46).
Patient values and preferences. The Work Group included 2
people living with CKD. These members’ unique perspectives
and lived experience, in addition to the Work Group under-
standing of patie nt preferences and priorities, informed de-
cisions about the strength of the recommendations. A
systematic review of qualitative studies on patient priorities
and preferences was not undertaken for this guideline.
Resources and other costs. Healthcare and non–healthcare
resources, including all inputs in the treatment management
pathway, were considered in grading the strength of a
recommendation.
937
The following resources were
considered: direct healthcare costs, non–healthcare resources
(such as transportation and social services), informal
caregiver resources (e.g., time of family and caregivers), and
changes in productivity. No formal economic evaluations,
including cost-effectiveness analysis, were conducted.
Practice points. In addition to graded recommendations,
KDIGO guidelines now include “practice points” to help
healthcare providers better evaluate and implement the guid-
ance from the expert Work Group. Practice points are consensus
statements about a specific aspect of care and supplement rec-
ommendations. These were developed when no formal sys-
tematic evidence review was undertaken or there was
insufficient evidence to provide a graded recommendation.
Practice points represent the expert judgment of the guideline
Work Group, and they may be based on limited evidence.
Practice points were sometimes formatted as a table, a figure, or
an algorithm to make them easier to use in clinical practice.
Format for guideline recommendations. Each guideline
recommendation provides an assessment of the strength of
the recommendation (Level 1, “we recommend” or Level 2,
“we suggest”) and the overall certainty of the evidence (A, B,
C, D). The recommendation statemen ts are followed by Key
information (Balance of benefits and harms, Certainty of the
evidence, Values and preferences, Resource use and costs,
Considerations fo r implementation), and Rationale. Each
recommendation is linked to relevant SoF tables. An under-
lying rationale may also support a practice point.
Table 47 | KDIGO nomenclature and description for grading recommendations
Grade
Implications
Patients Clinicians Policy
Level 1
“We recommend”
Most people in your situation would want
the recommended course of action, and
only a small proportion would not.
Most patients should receive the
recommended course of action.
The recommendation can be evaluated
as a candidate for developing a policy
or a performance measure.
Level 2
“We suggest”
The majority of people in your situation
would want the recommended course
of action, but many would not.
Different choices will be appropriate for
different patients. Each patient needs
help to arrive at a management
decision consistent with their values
and preferences.
The recommendation is likely to require
substantial debate and involvement of
stakeholders before policy can be
determined.
KDIGO, Kidney Disease: Improving Global Outcomes.
Table 48 | Determinants of the strength of recommendation
Factors Comment
Balance of benefits and harms The larger the difference between the desirable and undesirable effects, the more likely a strong recommendation
is provided. The narrower the gradient, the more likely a weak recommendation is warranted.
Certainty of the evidence The higher the certainty of evidence, the more likely a strong recommendation is warranted. However, there are
exceptions for which low- or very low-certainty evidence will warrant a strong recommendation.
Values and preferences The more variability or the more uncertainty in values and preferences, the more likely a weak recommendation is
warranted. Values and preferences were obtained from the literature, where possible, or were assessed by the
judgment of the Work Group, when robust evidence was not identified.
Resources and other costs The higher the costs of an intervention—that is, the more resources consumed—the less likely a strong
recommendation is warranted.
www.kidney-international.org methods for guideline development
Kidney International (2024) 105 (Suppl 4S), S117–S314 S281

Limitations of the guideline development process. Two peo-
ple living with diabetes and CKD were members of the Work
Group and provided invaluable perspectives and lived expe-
riences for the development of these guidelines. However, in
the development of these guidelines, no scoping exercise with
patients, searches of the qualitative literature, or formal
qualitative evidence synthesis examining patient experiences
and priorities were undertaken. As noted, although resource
implications were considered in the formulation of recom-
mendations, no economic evaluations were under taken.
methods for guideline development www.kidney-international.org
S282
Kidney International (2024) 105 (Suppl 4S), S117–S314

Biographic and disclosure information
Adeera Levin, MD, FRCPC (Work
Group Co-Chai r), is a professor of
medicine, Head of the Division of
Nephrology at the University of
British Columbia, and consultant
nephrologist at Providence Health
Care/St Paul’s Hospital, in Vancouver
Canada.
She is the Executive Director of
the BC Renal Agency, which oversees the care, planning, and
budgets for kidney services in the province of British
Columbia.
She is active in international activities across the spec-
trum of kidney activ ities and has ser ved in leadership roles at
the International Society of Nephrology (ISN), most recently
as Presiden t (2015–2017). S he was one of the founding
members of the Declaration o f Istanbul Custodian Group
(DICG) and ser ved as one of the first Co-Chairs of that
group. Sh e has been active in both ISN a nd DICG con-
cerning advocacy for patient rig hts for equitable access to
care, and in the prevention of exploitation of vulnerable
populations.
Her major research interests include nontraditional risk
factors for CVD in people with CKD and progression of CKD
variability, as well as models of care. She has over 600 peer-
reviewed publications and numerous book chapters. She is the
Principal Investigator on a large national Strategy for Patient-
Oriented Research (SPOR) network grant Can-SOLVE CKD
focusing on patient-oriented research. She collaborates with
investigators across Canada and internationally.
She has received numerous teaching and research awards
from Canadian Society of Nephrology, Kidney Foundation of
Canada, and British Columbia Health Research Institute, and
was inducted as a fellow into the Canadian Academy of
Health Sciences. For her contributions to the life of Cana-
dians, she was awarded the highest civilian honor, the Order
of Canada in 2015.
AL reports receiving consultancy fees from AstraZeneca*,
Bayer*, Janssen*, Novo Nordisk*, OccuRx*, and Otsuka*;
research support from AstraZeneca*, Boehringer Ingelheim*,
Canadian Institutes of Health Research (CIHR)*, Glaxo-
SmithKline*, National Institutes of Health (NIH)*, and
Otsuka*; speaker honoraria from AstraZeneca*, Bayer*, and
Boehringer Ingelheim*; and funding for the development of
educational presentations for AstraZeneca*, Bayer*, Boeh-
ringer Ingelheim*, and Novo Nordisk*.
*Monies paid to institution.
Paul E. Stevens, MB, FRCP, RCPathME
(Work Group Co-Chair), is consultant
nephrologist and medical examiner at
East Kent Hospitals University National
Health Service (NHS) Foundation Trust,
Kent and Canterbury Hospital in the UK.
He was appointed as Consultant Physician
and Nephrologist to the Royal Air Force
in 1990, returning to the NHS in April
1995 as Clinical Director of the Kent Kidney Care Centre,
implementing a program of modernization and development
and establishing a predominantly clinical research program in
kidney disease. He has served on several national and college
committees, is a former President of the British Renal Society,
and was an advisor to the Department of Health for both kidney
disease and national implementation of eGFR reporting. His
interest in guideline development began with commissioning
guidance for the development of kidney services and the first UK
CKD guideline in 2005. He served as clinical advisor and chair to
several of the UK National Institute for Health and Care Excel-
lence (NICE) Clinical Guidelines, was a member of the UK
consensus panel for management of AKI, and chaired the NICE
CKD topic expert reference group and the production of NICE
Quality Standards in CKD. He is the current treasurer of the
Kidney Disease: Improving Global Outcomes (KDIGO) Execu-
tive Committee and was privileged to have co-chaired the
KDIGO 2012 Clinical Practice Guideline for the Evaluation and
Management of Chronic Kidney Disease.
PES declared no competing interests.
Sofia B. Ahmed, MD, MMSc, FRCPC,
is a professor in the faculty of medicine
and dentistry at the University of
Alberta and the University of Alberta
Chair in Sex and Gender. Dr. Ahmed
completed her MD and internal med-
icine residency at the University of
Toronto and a nephrology fellowship
at Brigham and Women’s and Massa-
chusetts General Hospitals in Boston. She completed her mas-
ter’s in medical sciences at Harvard University. The recipient of
the 2022 Hypertension Canada Senior Investigator Award, the
2021 Canadian Medical Association May Cohen Award for
Women Mentors, and a 2020 American Society of Nephrology
Distinguished Mentor Award, Dr. Ahmed is strong proponent of
the importance of mentorship and fostering excellence in the
next generation of researchers.
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S283

Dr. Ahmed is clinician-scientist with a focus on sex and
gender differences in human kidney and cardiovascular phys-
iology and clinical outcomes. She is the Chair of the Canadian
Institutes of Health Research Institute of Gender and Health
Advisory Board, a member of the Canadian Medical Associa-
tion Journal Governing Council and the President-Elect for the
Organization for the Study of Sex Differences.
SBA reports receiving research support for CIHR*, Heart and
Stroke Foundation*, and NIH*; being a member of the CIHR
Institute of Gender and Health Ad visory Board, the Canadian
Medical Association Journal Governance Council (volunteer), the
Data Safety Monitoring Board Member for Adolescent Type 1
diabetes Treatment with SGLT2i for hyperglycEMia & hyPer -
filTration Trial (ATTEMPT) trial (trial sponsored by the CIHR
and J uvenile Diabetes Research Foundation Canada) (volunteer);
and serving as President-Elect, Organization for the Study of Sex
Differences (volunteer).
*Monies paid to institution.
Juan Jesus Carrero, Pharm, PhD
Pharm, PhD Med, is a professor of
cardio-renal epidemiology at Kar-
olinska Institutet. His research in-
volves the analysis of large routine-
care databases with the goal to
improve the identification and man-
agement of people with CKD.
Juan Jesus has published over 500
original publications on var ious as-
pects of the epidemiology of CKD, w ith emphasis on modifiable
risk factors: diet, lifestyle, processes of care, and inappropriate
use of medications. Juan Jesus has served in previous clinical
guidelines from KDIGO, KDOQI, and the European Society for
Clinical Nutrition and Metabolism (ESPEN) and currently
serves as co-director of the educational outreach program at the
International Society of Renal Nutrition and Metabolism
(ISRNM). He has received the Research Excellence Award of the
European Renal Association (ERA) and the Kopple award of the
US National Kidney Foundation (NKF).
JJC reports receiving research support from Amgen, Astellas,
AstraZeneca, Boehringer Ingelheim, Merck Sharp and Dohme,
Novo Nordisk, and Vifor Pharma; speaker honoraria from A bbott,
Baxter, and Fresenius K abi; and serving as a board member for
AstraZeneca, Baxter , F resenius K abi, and GlaxoSmithKline.
Bethany Foster, MD, MSCE, is a
professor of pediatrics, Chair of the
Department of Pediatrics at McGill
University and Pediatrician-in-C hief
at the McGill University Health
Centre. She is a pediatric nephrolo-
gist and a clinical epidemiologist
with a primary research interest in
the long-term outcomes of children
and young adults with kidney transplants. Dr. Foster has been
funded by CIHR and NIH to study immunosuppressive
medication adherence and graft outcomes in adolescent and
young adult kidney transplant recipients, whom she has
identified to be at particularly high risk of graft loss. She has
also highlighted important differences in kidney transplant
outcomes by recipient sex, the magnitude and direction of
which vary by recipient age and by donor sex. Dr. Foster has
over 110 peer-reviewed publications and is an Associate Ed-
itor of the international journal Transplantation . She
contributed to the KDOQI Clinical Practice Guideline for
Nutrition in Children with CKD: 2008 Update and to the
KDIGO 2020 Clinical Practice Guideline on the Evaluation
and Management of Candidates for Kidney Transplantation.
She is also Chair of The Transplantation Society’s Women in
Transplantat ion initiative.
BF reports receiving research supp ort from CIHR* and NIH*,
and serves as Chair of the Women in Transplantation
Initiative of The Transplantation Society.
*Monies paid to institution.
Anna Francis, BSci, MBBS, FRACP,
CF, MMed, PhD, is a clinician
researcher at the University of
Queensland and at Queensland
Children’s Hospital, Australia. She
has broad clinical experience in pe-
diatric nephrology and young adult
CKD care with clinical appointments
at Queensland Children’s Hospital
and the Mater Young Adult hospital. Dr. Francis was awarded
a prestigious Churchill Fellowship, traveling to Germany,
England, and the US to explore transition programs to adult
care for young kidney transplant recipients; she has set up the
pediatric kidney transition service and is co-lead in the young
adult kidney transplant clinic in Queensland. Dr. Francis has
published over 50 articles on research areas such as quality of
life of children with CKD and long-term outcomes for chil-
dren with CKD, including transplantation outcomes and
survival. She is an associate editor at Kidney International
Reports and is on the editorial board of Kidney International ,
Journal of Nephrology, and Transplant International. She was a
member of the inaugural ISN Emerging Leaders Program.
AF declared no competing interests.
Rasheeda K. Hall, MD, MBA, MHS,
is an associate professor of medicine
in the Division of Nephrology at
Duke University School of Medicine,
Durham, NC, USA. Dr. Hall received
a medical degree from Vander bilt
University School of Medicine. She
trained in internal medicine and
nephrology at Duke University. She
biographic and disclosure information www.kidney-international.org
S284
Kidney International (2024) 105 (Suppl 4S), S117–S314

practices nephrology at the Durham Veterans Affairs
Healthcare System, leading a geriatric nephrology clinic.
This innovative clinic incorporat es geriatric assessment to
inform CKD management and dialysis decision-making
conversations. Her research focuses on the integ ration of
geriatr ic principles into kidney care settings. Her research
has also included observational cohort studies of physical
function, frailty, and resilience; qualitative studies on
quality of life and geriatric care, pharmacoepidemiology of
potentially inappropriate medications (PIMs), deprescribing
intervention development, and geriatric models of care. She
recently started the Kidney Disease Aging Research
Collaborative, a US-based initiative to lay the foundation
for collaboration across multiple institutions on geriatric
nephrology research. She also serves on the editorial board
for American Journal of Kidney Diseases, Clinical Journal of
the American Society of Nephrology, and Journal of the
Amer ican Geriatrics Society.
RKH reports receiv ing consultancy fees from Bayer and
United Health Group; research support from American
Society of Nephrology Foundation for Kidney Research*,
National Institute on Aging*, and Robert Wood Johnson
Foundation*; and serving on the Advancing Kidney
Health through Optimal Medication Management
(AKHOMM).
*Monies paid to institution.
William G. Herrington, MA, MBBS,
MD, FRCP, is professor of trials and
epidemiolog y of kidney disease at the
Nuffield Department of Population
Health, University of O xford and a
practicing Honorary Consultant
Nephrologist at Oxford Kidney Unit.
He jointly leads the Renal Studies
Group, which he joined in 2010 as a
Clinical Research Fellow and trained on landmark kidney
trials (SHARP, 3C, and UKHARP3).
He is Chief Investigator of the EMPA-KIDNEY trial,
which tested the effects of empagliflozin 10 mg versus
placebo on cardiorenal outcomes in 6609 people with CKD
with and without diabetes. He is on a number of clinical
practice guideline working groups and co-chairs the UK
Kidney Association guideline group responsible for rec-
ommendations on the use of SGLT-2 inhibitors in adults
with kidney disease. He is also interested in trial method-
ology and has chaired the UK Renal Trials Network since
2020.
His epidemiological research aims to better understand the
key determinants of kidney disease development and pro-
gression (and its associated complications) using observations
from large blood-based prospective cohorts across a wide
range of different populations. He has a particular focus on
adiposity and its related risk factors, and how these may
interlink to also cause cardiovascular disease. He is also
focusing on how novel blood and urine biomarkers could
better assess effects of treatments on the kidney and predict
progression.
WGH reports receiving research sup port from Boehringer
Ingelheim* and Eli Lilly*, and serving on the Data Moni-
toring Committee for Bayer (unpaid).
*Monies paid to institution.
Guy Hill was diagnosed with IgAN
in 1996 at the age of 35 and was in
kidney failure within 2 years. After a
2-year period of peritoneal dialysis,
he had a transplant in 2001. This
lasted until 2008 and then he did 4
years of home hemodialysis before a
further transplant in 2012 that failed
to work for a further 9 months. Once
awake, this transplant lasted until 2016 and then followed a
further 4 years of home hemodialysis, which ended with a live
transplant from his brother in 2019, which is working suc-
cessfully today.
He has taken an active interest in patient advocacy and
support since 1999, mainly locally with his Manchester Kid-
ney Patient Association of which he is chair. He is also on the
Patient Advisory Group for the National Kidney Charity,
Kidney Care UK. He has also been patient representative on
several NICE assessments of new devices and drugs for people
with CKD.
He has attended a full range of kidney conferences and
professional discussion groups at a local, regional, and na-
tional level on health service organizations that affect kidney
patients.
His contact with many patients and professionals from all
areas of the kidney service has given him a broad knowledge
of all stages of kidney care and its challenges.
GH declared no competing interests.
Lesley A. Inker, MD, MS, FRCP (C),
is professor of medicine at Tufts
University School of Medicine, and
an attending physician and Medical
Director of the Kidney and Blood
Pressure Center in the Division of
Nephrology at Tufts Medical Center.
Dr. Inker’s primary research in-
terests are in kidney function mea-
surement and estimation, alternative endpoints for clinical
trials of kidney disease progression, and epidemiology and
outcomes related to CKD. She is co-director of the Chronic
Kidney Disease Epidemiology collaboration (CKD-EPI). Dr.
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S285

Inker has worked with NKF leadership on multiple public
health initiatives for CKD care in the United States, including a
member of the recent joint NKF-American Societ y of
Nephrology (ASN) task force on reassessing use of race in
diagnosis of CKD. Dr. Inker is the inaugural chair of the steering
committee for the NKF Patient Network. She has chaired, or led
analytical teams, for several scienti fic workshops related to
surrogate endpoints for CKD progression. She is an investigator
on several trials of kidney disease progression. She has also
received many honors and awards, including the Garabed
Eknoyan Award from the NKF, the ASN mid-career research
award, and the Milton O. and Natalie V. Zucker Pr ize.
LAI reports receiving consultancy fees from Diamtrix and
Tricida*; and research support from Chinook*, NIH*, Na-
tional Kidney Foundation*, Otsuka and Reata.
*Monies paid to institution.
Rümeyza Kazancıo
glu, MD, is the
president and a professor of nephrology
at Bezmialem Vakif U niv ersity
_
Istanbul,
Turkey . She received her medical
degree from Istanbul U niversity School
of Medicine,
_
Istanbul.
She served as a co uncil member of
ISN as well as the chair of East and
Central Europe Regional Board. She
was also a member of the International Society of Pe ritoneal
Dialysis Middle East chapter board. She currently chairs the ISN
fellowship c ommittee and is a member of both ISN and Turkish
Society of Nephr ology’s Renal Disaster Pr eparedness Working
groups. She also serves as a member at board of councilors at
DICG.
Dr. Kazancıo
glu is the editor-in-chief of Turkish Journal of
Nephrology.
Her main areas of interest are glomerular disease, home
therapies especially peritoneal dialysis, and disaster/conflict
medicine. She has participated in previous KDIGO Contro-
versies Conferences.
RK reports receiving speaker honorari a from Astellas* and
Baxter Healthcare*.
*Monies paid to institution.
Edmund Lamb, PhD, FRCPath, is
consultant clinical scientist and clin-
ical director of pathology at East Kent
Hospitals University NHS Trust,
Canterbury, Kent, UK. He has a
special interest in kidney disease and
undertook his PhD in kidney
research at St Bartholomew’s Hospi-
tal, London. His research interests
relate to the use of biochemical markers to diagnose and
monitor kidney disease, including the assessment of kidney
function using estimated GFR and cystatin C and the evalu-
ation of renal bone disease; he is coauthor of more than 100
peer-rev iewed papers in this area. He has been a member of
national and international guideline development groups
including NICE and KDIGO CKD guidelines and the
Department of Health initiative to roll out eGFR across En-
gland. He is a former editor-in-chief of Annals of Clinical
Biochemistry.
EL reports receiving research support from National Institute
of Health Research*.
*Monies paid to institution.
Peter Lin, MD, CCFP, is the director
of primary care initiatives at the Ca-
nadian Heart Research Centre. He
has a busy family practice in Toronto,
Canada. He is also a contributing
author to the Canadian Diabetes
Guidelines 2013 and 2018 on the
vascular protection section and an
associate editor for the Elsevier Web
Portal—Practice Update Primar y Care. Dr. Lin has lectured
extensively on diabetes and its complications, especially
CKD, and he has worked with KDIGO to help improve care
for people with CKD. He has also been tracking and
providing information on COVID-19 to the public since the
beginning of the pandemic. He reaches out to the public with
his role as a medical contributor to the Canadian Broad-
casting Corporation (CBC) which is the national news agency
in Canada.
PL reports receiving consultancy fees from AstraZeneca,
Bayer, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, and
Novo Nordisk; speaker honoraria from AstraZeneca, Bayer,
Boehringer Ingelheim , Eli Lilly, Janssen, Merck, and Novo
Nordisk; funding for development of educational pre-
sentations for AstraZeneca, Bayer, Boehringer Ingelheim, Eli
Lilly, Janssen, Merck, and Novo Nordisk; and serv ing as the
Associate Editor of Elsevier Online Practice Update Primary
Care.
Magdalena Madero, MD, is a pro-
fessor of medicine and the chief of
nephrology at the National Heart
Institute in Mexico City. She was
trained in Internal Medicine at St
Elisabeth’s Medical Center in Boston,
MA, and then underwent her
nephrology training at Tufts Medical
Center also in Boston, MA. She went
back to Mexico City in 2007 where she joined the nephrology
staff at the National Heart Institute and became the Head of
the Nephrology Division in 2011. Dr. Madero’s research in-
terests include CKD progression, complications, and
biographic and disclosure information www.kidney-international.org
S286
Kidney International (2024) 105 (Suppl 4S), S117–S314

outcomes, CKD of unknown origin and hemodialysis. She
has over 100 publications and 7000 citations. She was awar-
ded the Miguel Aleman award in 2015, given to the most
outstanding young researcher in the country. As part of her
educational activities, she runs the largest kidney fellowship
programs in the country at the National Heart Institute
(affiliated to the main National Mexican University [UNAM])
in addition to teaching the nephrology course at the under-
graduate Panamerican University medical school. She enjoys
patient care and is active in taking care of people with CKD.
She was the former President of one of the Mexican Societies
of Nephrology (IMIN) and served as International Editor for
the American Journal of Kidney Diseases (2016–2021), as a
member of the KDIGO Executive Committee (2018–2021).
She served as the Chair for the ISN for Latin America and the
Caribbean (2019–2023) and is a council member for the
Society of Peritoneal Dialysis (2022–2024). She will become
an associate editor for Journal of the American Society of
Nephrology in 2024.
MM reports receiving consultancy fees from AstraZeneca, Bayer,
and Boehringer Ingelheim; research support from AstraZeneca*,
Bayer*, Boehringer Ingelheim*, Renal Research Institute*, and
Tricida*; speaker honoraria and travel from AstraZeneca; and
funding for expert testimony for AstraZeneca, Bayer, and
Boehringer Ingelheim.
*Monies paid to institution.
Natasha McIntyre, PhD, is a clini-
cian scientist in London, Ontario.
She qualified as a nurse in 1991 in
London, UK, where she specialized
in nephrology nursing and worked in
the NHS , holding a number of senior
nursing leadership roles, until mov-
ing to Canada in 2014.
Whilst in the UK, she completed
her PhD at the University of Nottingham, funded by a
research fellowship from Kidney Research UK and the British
Renal Society, focusing on people in primary care with CKD
G3, recruiting and following a cohort of 1741 people (the
Renal Risk in Derby cohort study). Together with post-
doctoral work, she has disseminated discoveries and co-
authored scientific papers in a number of peer-reviewed
nephrology journals.
Throughout her career she has been actively involved in
quality improvement for people with CKD or AKI and has
experience of employing key quality improvement meth-
odologies in healthcare settings on a local, national, and
international scale; working with the NICE and the Na-
tional Patient Safety Agency in the UK and the Dialysis
Outcomes and Patient Patterns Study (DOPPS) global
research collaborative.
More recently she has been involved in the development of
the Centre for Quality, Innovation and Safety, in London,
Ontario as well as obtaining funding to research the evolution
of virtual healthcare during and after pandemic and how this
may impact on future models of healthcare.
NM declared no competing interests.
Kelly Morrow, MS, RDN, CD,
FAND, is a registered dietitian
nutritionist and fellow of the
Academy of Nutrition and Dietetics.
Having autosomal dominant poly-
cystic kidney dise ase as well as a
kidney transplant has shaped her
interest in nutrition and commit-
ment to providing compassionate
care for her kidney patients. She
has been on the faculty at Bastyr University since 2002
where she has supervised clinical rotations in the Uni-
versity’s community health clinic and taught in the De-
partments of Nutrition and Exercise Science, Naturopathic
Medicine, Midwifery and Acupuncture and East Asian
Medicine. She is an affiliate dietitian with the Osher Center
for Integrative Medicine at the University of Washington
Department of Family Medicine, is a past Chair of Di-
etitians in Integ rative and Functional Medicine through the
Academy of Nutrition and Dietetics, and is a Co-Editor of
Krause and Mahan’s Food and the Nutrition Care Process
textbook. She has published and been an invited speaker
on topics related to integrative nutrition and dietary sup-
plements and currently practices clinical nutrition in Seat-
tle, Washington.
KM declared no competing interests.
Glenda Roberts was an information
technology executive with 35þ years
of experience with the Global 100
corporations, like Microsoft and
others before joining the University
of Washington (UW) in 2018 as the
Director of External Relations & Pa-
tient Engagement for the UW Kidney
Research Institute and the UW
Center for Dialysis Innovation (CDI); and the Chief Opera-
tions and Strategy Officer for UW’s Justice, Equity, Diversity
and Inclusion Center for Transformative Research.
A passionate activist for research and people living with
kidney diseases, she has received numerous awards and
recognition for her work in kidney health. She was 1 of 2
patients who served on the National Kidney Foundation
(NKF)—American Society of Nephrology (ASN) Task Force:
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S287

Reassessing the Use of Race in Diag nosing Kidney Disease
that resulted in the removal of race from the estimated
glomerular filtration rate (eGFR) formula. Recently NKF
announced that Glenda is the most recent recipient of the
Celeste Lee Patient Engagement Award, the highest honor
given by NKF to a distinguished kidney patient who exem-
plifies NKF’s mission and Celeste’s legacy of putting patients
at the center of all aspects of healthcare through their
involvement with NKF and community partners. In 2022, the
ASN honored her with its highest award, the President’s
Medal. She was the 2023 “Accelerate Innovation” spokes-
person for the “We’re United 4 Kidne y Health” campaign,
which invites healthcare professionals to join the movement
to sh ift their focus from kidney failure to kidney health.
With her CDI team, she won a KidneyX Redesign Dialysis
Phase1prizefor“The A mbulatory Ki dney to Im prove
Vitality (AKTIV).” The Kidney Week 2021 Celeste Castillo
Lee Memorial Lecturer, Glenda also recei ved the Pres ident’s
Volun teer Service Awards from President Donald J. Trump
and President Joseph R. Biden, in 2020 and 2022,
respectively.
Glenda has been involved in a myriad of regional, national,
and international, transformative kidney healthcare initia-
tives. Many of these are focused on developing new innovative
treatments and therapies to make life better for people living
with kidney diseases and the cardio-kidney-metabolic syn-
drome. In addition to being involved with a number of
KDIGO (Kidney Disease: Improving Global Outcomes) ini-
tiatives, she serves on the Board of Directors for the Kidney
Health Initiative (KHI), a partnership between the US Food &
Drug Administration and ASN, whose mission is to catalyze
innovation and the development of safe and effective patient-
centered therapies for people liv ing with kidney diseases.
Glenda has been actively involved with and has a leadership
position in several research projects, including the Kidney
Precision Medicine Project (KPMP), the APOL1 Long-term
Kidney Transplantation Outcomes Network (APOLLO), the
BLOod Sugar Sensing On Maintenance dialysis (BLOSSOM),
the Biomarker Data Repository (BmDR) and numerous pa-
tient advisory committees supported by federal programs,
pharmaceutical companies, and other public and private
funders. Since 2018, she has authored/co-authored or been
featured in over 35 publications.
GR declared no competing interests.
Dharshana Sabanayagam, MD,
FRACP, is an adult nephrologist,
working as a Post-Graduate Fellow at
Westmead Hospital, Sydney,
Australia. She is also enrolled in a
Master of Philosophy with the Uni-
versity of Sydney, with a focus on
optimization of dialysis initiation in
people with kidney failure.
DS declared no competing interests.
Elke Schaeffner, MD, MSc, is a
board-certified nephrologist and an
epidemiolog ist at the Institute of
Public Health, Charité—Uni-
versitätsmedizin Berlin where she
holds a professorship for Nephrology
and Health Care Research. She
studied Medicine at the University of
Freiburg, Germany and obtained her
Master of Science in Epidemiology at the Harvard School of
Public Health, Boston , USA. Dr. Schaeffner ’s primary fields of
research are renal epidemiology and aging, with a particular
focus on CKD in an aging society as well as biomarkers for
assessing kidney function. She is principal investigator (PI) of
the “Berlin Initiative Study” a population-based cohort study
investigating the epidemiology of CKD in persons aged 70þ
over the course of several years. Dr. Schaeffner’s engagement
in education has made her one of the leading figures in
launching a new master’s degree program (MScPH) at the
Berlin School of Public Health where she is deputy director.
Since the beginning of 2022, Dr. Schaeffner has joined the
editorial board of AJKD as international editor. She was
awarded the ASN distinguis hed leader award in 2022. Also in
2022, Dr. Schaeffner was elected an executive board member
of the German Societ y of Nephrology.
ES reports receiving consultancy fees from AstraZeneca;
research support from Bayer AG* and E.N.D.I. Stiftung*;
speaker honoraria from Verband dt. Nierenzentren; and
serving on the Executive Board of the German Society of
Nephrology and the Editorial Board of National Kidney
Foundation.
*Monies paid to institution.
Michael Shlipak, MD, MPH, is the
co-founder and scientific director of
the Kidney Health Research Collab-
orative (KHRC) at the University of
California, San Francisco (UCSF)
and the San Francisco Veterans Af-
fairs Healthcare System (SFVAHCS),
where he also serves as the associate
chief of medicine for research
development. At SFVAHCS, Dr. Shlipak previously served as
the division chief for General Internal Medicine from 2004 to
2018; at UCSF, he is professor of medicine, epidemiology &
biostatistics. Dr. Shlipak’s training comprised a degree in His-
tory from Dar tmouth College, followed by Harvard Medical
School, and the Harvard School of Public Health. He completed
internal medicine residency and a General Internal Medicine
fellowship at UCSF. His research activities involve the detection
and the determinants of kidney disease, and its association with
adverse outcomes, including cardiovascular disease. He has
particularly been a pioneer on the use of cystatin C as a novel
indicator of kidney function and its potential to improve un-
derstanding of kidney disease epidemiology and clinical care.
biographic and disclosure information www.kidney-international.org
S288
Kidney International (2024) 105 (Suppl 4S), S117–S314

For that body of work, Dr. Shlipak was awarded the John
Blair Barnwell Award in 2018 from VA Clinical Science
Research and Development Service. Much of his current
research is focused upon novel diagnostic opportunities that
utilize urine proteins to characterize chronic and acute kidney
diseases. Dr. Shlipak’s research has been continuously funded
by NIH grants for the past 22 years, in addition to research
grants from VA Health Services Research and Development,
the Robert Wood Johnson Foundation, the American Heart
Association, and the American Federation for Aging Research.
Dr. Shlipak is the author of over 500 peer-reviewed manu-
scripts. In addition, Dr. Shlipak was a writing member of the
KDIGO 2012 Clinical Practice Guideline for the Evaluation
and Management of Chronic Kidney Disease, and he is a
member for the 2024 update of this guideline. He also served
as Co-Chair and lead author for the KDIGO 2019 Contro-
versies Conference entitled “Early Identification and Inter-
vention in CKD”.
MS reports receiv ing research support from Bayer*, NIH
(NHLBI, NIA, NIDDK)*, VA Health Services Research &
Development*, and VA Clinical Science Research & Devel-
opment*; speaker honoraria from AstraZeneca, Bayer, and
Boehringer Ingelheim; and funding for expert testimony for
Hagens Berman International Law Firm.
*Monies paid to institution.
Rukshana Shroff, MD, FRCPCH,
PhD, is a professor of pediatric
nephrology at Great Ormond Street
Hospital for Children and University
College London, UK. Her research
focuses on bone and cardiovascular
disease in childhood CKD, aiming to
improve outcomes for children on
dialysis. She has led several
international multicenter tria ls in the field.
Dr. Shroff is co-editor for the 8th edition of Pediatric
Nephrology, the definitive textbook in our field. She is the
Scientific Chair for the European Society for Pediatric
Nephrology (ESPN) meeting in 2023. She has received a
prestigious senior fellowship from the National Institute for
Health Research, served as a member of the KDIGO Executive
Committee, and participated in international guideline
committees through KDIGO, NICE, and ESPN. She is chair
of the ESPN Dialysis working group and represents pediatric
dialysis at the ERA. She has developed the Paediatr ic Renal
Nutrition Taskforce, and co-chairs the ISN Sister Renal
Centre Program.
RS reports receiving consultancy fees from AstraZeneca* and
Fresenius Medical Care*; research support from Fresenius
Medical Care* and Vitaflo*; speaker honoraria from Amgen
and Fresenius Medical Care.
*Monies paid to institution.
Navdeep Tangri, MD, PhD,
FRCP(C), is an atten ding physician
and professor in the Division of
Nephrology, Department of Internal
Medicine and the Rady Faculty of
Community Health Sciences at the
University of Manitoba. Dr. Tangri’s
research program is clinical, trans-
lational, and focused on improving
clinical decision-making for people with advanced CKD. He
developed and validated the Kidney Failure Risk Equation
(KFRE) to predict the need for dialysis in patie nts with CKD
and is presently engaged in multiple validation and imple-
mentation efforts to increase the uptake of the KFRE.
In addition, Dr. Tangri is conducting a large prospective
study on frailty, physical, and cognitive function in advanced
CKD, as well as leading a multination al randomized trial on
the safety and efficacy of new therapies in this population. He
has published over 350 manuscr ipts, presented at multiple
national and international scientific meetings, and is a
recipient of the CIHR New Investigator Award and a CIHR
Foundation grant.
NTreports receiving consultancy fees from AstraZeneca, Bayer,
Boehringer Ingelheim, GlaxoSmithKline, Janssen, Otsuka,
ProKidney, and Roche; research support from AstraZeneca*,
Bayer*, Boehringer Ingelheim*, and Janssen*; funding for
development of educational presentations for AstraZeneca;
having stock/stock options from Clinpredict, Klinrisk, Mar-
izyme, ProKidney, Pulsedata, and Quanta; and a patent for a
microfluidic device for measuring ACR at po int of care.
*Monies paid to institution.
Teerawat Thanachayanont, MD,
MSc, is a senior nephrologist at
Bhumirajanagarindra Kidney Insti-
tute, Bangkok, Thailand. He gradu-
ated Doctor of Medicine from
Mahidol University, Thailand, and
did internal medicine training at
Siriraj Hospital, Mahidol University,
Thailand. He has postgraduate
training in Nephrology at the University of British Columbia,
Canada, and has done a 1-year training in independent
dialysis at the University of British Columbia, Canada.
He is currently the head of the CKD clinic and Peritoneal
Dialysis unit of Bhumirajanagarindra Kidney Institute Hos-
pital. His clinical work includes management of predialysis
CKD, peritone al dialysis, and hemodialysis patients. He also
initiated the in-center nocturnal hemodialysis program in
Thailand. For academic work, he is an adjunct clinical
instructor at Chulabhorn Royal Academy, Thailand, and a
lecturer at the Nephrology Society of Thailand and Royal
College of Family Physicians of Thailand.
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S289

His research interests focus on the prevention and man-
agement of CKD in both urban and rural settings and
improving dialysis-related outcomes in people receiving
peritoneal dialysis and hemodialysis. His recent research on
integrated care models for CKD management in rural areas of
Thailand has made a great contribution to the CKD man-
agement healthcare policy in Thailand. He and his research
team are continuing implementation of a national integrated
care model program for CKD management in the rural areas
of Thailand.
TT declared no competing interests.
Ifeoma Ulasi, MBBS, FWACP, PGD,
MSc, is a professor of medicine at the
College of Medicine, University of
Nigeria. She has affiliations with 2
teaching hospitals where she is involved
in patient management, training med-
ical students, student nurses, post-
graduate students, and resident
doctors. She is also active in various
research fields such as epidemiology, sociobehavioral studies,
genetic/genomic research, clinical trials, and interventions.
Furthermore, she serves as the chief physician of the
University of Nigeria Nsukka Centre of Excellence for Clinical
Tr ials, the Site PI for the H3Africa Kidney Disease Network
Project, and the PI of International Diabetes Federation-
sponsored clinical trials in gestational diabetes. In addition,
she is the Deputy Chair of ISN Advocacy Working Group
(AWG) and a former member of ISN ExCom (2021–2023), a
member of The Transplantation Society (TTS) Ethics Com-
mittee, and the WHO Taskforce on Organ Donation and
Transplanta tion. Dr. Ulasi also serves as the Coordinator for
the West Africa College of Medicine Post-graduate College
subspecialty Examinations in Nephrology (2016–2020) and as
the President of the Nigerian Association of Nephrolog y
(2018–2020). Lastly, she is an international adviser at the
Royal College of Physicians, London.
IU reports receiving speaker honoraria from AstraZeneca and
Boehringer Ingelheim.
Germaine Wong, MD, PhD, is a
transplant nephrologist, Director of
Western Renal Service at Westmead
Hospital, Professor of Clinical Epide-
miology, NHMRC Leadership Fellow
at the University of Sydney. She is the
current co-chair of the Women in
Transplantation. She has an interna-
tionally recognized track record in
transplant epidemiology, cancer and transplantation, social
ethics in organ allocation, decision analytical modeling, health
economics, and quality of life studies in transplant recipients.
GW declared no competing interests.
Chih-Wei Yang, MD, is the Vice
President of Chang Gung University,
and he is a leader in the field of
medicine and nephrology in Taiwan.
He has held numerous roles at Chang
Gung University and Chang Gung
Memorial Hospital, includ ing serving
as Dean of the College of Medicine
and founding the Chang Gung
Kidney Research Center. His research, particularly focused on
infection-related kidney diseases like leptospirosis kidney
disease, has earned him accolades such as the Distinguished
Research Award from the National Science Council and the
Outstanding Contribution Award from the Taiwan Society of
Nephrology and the National HealthCare Quality Award.
Beyond his local impact, Dr. Yang has made significant
contributions on a global scale. He has actively participated in
organizations like the Taiwan Society of Nephrology, Asian-
Pacific Societ y of Nephrology, and the ISN, where he repre-
sented the North and East Asian region, served on various
committees, Councilor and Executive Committee Member.
He is currently the Chair of the ISN Sister Renal Center
Program and co-Chair of the ISN-TTS Sister Transplant
Program.
His dedication to advancing research, education, and in-
ternational collaboration in nephrology has solidified his
position as a leader in the field, contributing continuously to
improve kidney health in Taiwan and worldwide.
C-WY declared no competing interests.
Luxia Zhang, MD, MPH, is the
deputy dean of the National Institute
of Health Data Science at Peking
University, China, and Professor in
the Renal Division of Peking Uni-
versity First Hospital, China. She
obtained her MD degree at Peking
University and her MPH degree at
Harvard School of Public Health.
Her research focuse s on prevalence, risk factors, intervention,
and management of kidney disease in China. Most of her
work provides first-hand information on kidney disease in
China and has gained wide attention internationally. During
the last several years, her study interests have been expanded
to the management of major noncommunicable chronic
diseases by leveraging the power of big data and machine
learning. Her studies have been published in top medical
journals including New England Journal of Medicine, the
Lancet, and British Medical Journal. Dr. Zhang was named on
the list of the “World’s Top 2% Scientists 2020" from Stanford
University and the “2020 China Highly Cited Scholars” list
from Elsevier. She is the Vice President of Health Data
Application and Management Committee, Chinese Hospital
Association; Deputy Editor of Health Data Science (a Scien ce
Partner Journal); member of the Lancet Digital Health
biographic and disclosure information www.kidney-international.org
S290
Kidney International (2024) 105 (Suppl 4S), S117–S314

International Advisory Board; and member of Editorial
Boards of Clinical Journal of the American Society of
Nephrology and American Journal of Kidney Diseases.
LZ reports receiving research support from AstraZeneca* and
Bayer*
*Monies paid to institution.
KDIGO Chairs
Michel Jadoul, MD, received his MD
degree in 1983 at the Université
Catholique de Louvain (UCLouvain),
Brussels, Belgium. Dr. Jadoul trained
in internal medicine and nephrology
under the mentorship of Professor
Charles van Ypersele de Strihou. He
has served as chair at the Department
of Nephrology of the Clini ques
Universitaires Saint-Luc (2003–2023) and is currently a full
clinical professor at UCLouvain. Dr. Jadoul’s clinical activities
focus on the follow-up of hemodialysis and CKD patients, and
his main research interests include
b
2-microglobulin amyloid-
osis, hepatitis C, and other complications (e.g., falls, bone
fractures, and sudden death) in hemodialysis patients, as well as
cardiovascular complications after kidney transplantation and
various causes of kidney disease (e.g., drug-induced).
Dr. Jadoul has coauthored over 350 scientific papers, most
of them published in major nephrology journals. He is
currently serving as an associate editor of Nephrology Dialysis
Transplantation, and he is also a country co-investigator for
DOPPS (2001–present). In 2008, he received the Interna-
tional Distinguished Medal from the US NKF. He was pre-
viously a member of the ERA Council (2013 – 2016). Presently,
Dr. Jadoul is a KDIGO Co-Chair.
MJ reports receiving consultancy fees from Astellas*, Astra-
Zeneca*, Bayer*, Boehringer Ingelheim*, Cardiorenal*, CSL
Vifor*, Fresenius Medical Care Asia Pacific*, GlaxoSmith-
Kline*, Mundipharma*, and Vertex*; grants/research support
from Amgen and AstraZeneca*; speaker honoraria for
AstraZeneca*, Bayer*, and Boehringer Ingelheim*; funding
for exper t testimony from Astellas* and Stada-Eurogenerics*;
travel support from AstraZeneca*.
*Monies paid to institution.
Morgan E. Gr ams, MD, PhD, MHS,
is the co-director of the New York
University Divi sion of Precision
Medicine, a multidisciplinary
research unit that aims to produce
evidence to inform the d eliver y of
high-quality, equitable patient care
responding rapidly to changes in
healthcare guidelines, delivery,
safety, and regulation. A practicing nephrolog ist, PhD-
trained epidemiolog ist, and the Susan and Morris Mark
Professor of Medicine and Population Health at New York
University,Dr.GramsisCo-PrincipalInvestigatorofthe
Chronic Kidney Disease Prognosis Consortium (CKD-PC),
a consortium of over 30 million participants, 100 cohort s,
and 250 investigators from around the globe. I n this role, Dr.
Grams and the CKD-PC team focus on developing, testing,
and implementing analy tic strateg ies to answer clinically
meaningful questions using as much of the world’s data on
kidney measures and o utcomes as possible. She also leads
effortstointegratemultimodalomicsdataastheyrelateto
kidney disease. She was the winner of the Young Investig ator
Awardin2018givenbytheASN/AmericanHeartAssocia-
tion Kidney Council, the top award for investigators under
45yearsofage,andsheisamemberoftheAmericanSociety
of Clinical Investigation. She attended medical school at
Columbia University and completed her nephrology
fellowship at Johns Hopkins University. She is also a C o-
Chair of KDIGO.
MEG declared no competing interests.
Methods Committee Representative
Bertram L. Kasiske, MD, FACP, did
his undergraduate training at Mich-
igan State University, East Lansing,
Michigan. He received his medical
degree from the University of Iowa,
Iowa City, Iowa. He completed In-
ternal Medicine residency, and
fellowship training in Nephrology, at
Hennepin County Medical Center,
an affiliate hospital of the University of Minnesota in Min-
neapolis. He is former deputy director of the United States
Renal Data System, former editor-in-chief of the American
Journal of Kidney Diseases, former Co-Chair of KDIGO,
former Director of Nephrology at Hennepin County Medical
Center, and former Director of the Scientific Registry of
Transplant Recipients. He is professor of medicine at the
University of Minnesota, and he is currently President of the
Board of Trustees of the CADASIL Association, Inc., a patient
advocacy group for the rare disease cerebral autosomal
dominant arteriopathy with subcortical infarcts and leu-
koencephalopathy (CADASIL).
BLK declared no competing interests.
Evidence Review Team
Karen A. Robinson, PhD, is a pro-
fessor in the Department of Medicine
at the Johns Hopkins University
School of Medicine with joint ap-
pointments in the Department of
Epidemiolog y and the Department of
Health Policy & Management at the
university’s Bloomberg School of
Public Health. Dr. Robinson con-
ducts systematic reviews and research on the use of ev idence
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S291

in making decisions. She is director of the Johns Hopkins
University Evidence-based Practice Center and, within the
EPC Program, serves as an Associate Editor and on the
Methods Steering Committee. For over 20 years, she has been
an active member of Cochrane, where she has been a sys-
tematic review author, a methods researcher as well as an
editor for 2 review groups (including the methodology rev iew
group). Within the Guidelines International Network she
served on the steering committees for 2 groups (Tech; North
America). Dr. Robinson received an MSc in health sciences
from the University of Waterloo, Ontario, and a PhD in
epidemiolog y from the Johns Hopkins Bloomberg School of
Public Health.
KAR declared no competing interests.
Lisa Wilson, ScM, is a senior research associate in Health
Policy and Management in the School of Public Health. She
has been with the Johns Hopkins University Evidence-based
Practice Center for more than 15 years and has managed over
20 syste matic reviews and several method projects. As a
member of the Evidence Review Team, she participated in all
aspects of the rev iew and took the lead in drafting synthesis
sections, conducting meta-analyses, and drafting evidence
profiles.
LW declared no competing interests.
Renee F. Wilson, MS, has worked
with the Johns Hopkins University
Evidence-based Practice Center
since July 2 004 as a senior research
program manager. She has extensive
experience in systematic review
methods (including development of
comprehensive literature se arch
strategies using multiple databases),
meta-analysis, qualitative synthesis, and coordination and
management of large multidisciplinar y, collaborative pro-
jects. Before working with KDIGO, she completed 3 large -
scale systematic reviews relevant to kidney disease focusing
on frequency and duration of hemodialysis and qualit y of
life asse ssment in a Medicare population w ith kidney failure;
comparative effects of different contrast m ed ia on cont rast-
induced nephropathy; and comparative effectiveness of
measures to prevent contrast-induced nephropathy. In
addition to working with the Evidence-based Practice Center
she worked on a project sponsored by Patient-Centered
Outcomes Research Institute developing methods for
guideline developers to use when writing guidelines for in-
dividuals with multiple chronic conditions. She was a co-
investigator on the Evidence Review Team.
RFW declared no competing interests.
Dipal M. Patel, MD, PhD, is an as-
sistant professor of medicine at the
Johns Hopkins University, Division
of Nephrology. She is a practicing
nephrologist with a research interest
in the implementation of patient-
reported outcomes and additional
person-centered practices in
nephrology care. She served as an
internal advisor to the Evidence Review Team.
DMP declares receiving research support from Edward S. Kraus,
MD Scholar Fund, National Kidney Foundation, and National
Institute on Minority Health and Health Disparities, Mid-Atlantic
Center for Cardiometabolic Health Equity (MACCHE).
Troy Gharibani, BS, BA, is a research
assistant at the Johns Hopkins
Bloomberg School of Public Health.
In 2022, he graduated from the Uni-
versity of Maryland with a Bachelor of
Science in Neurobiology and a Bach-
elor of Arts in English and joined the
Johns Hopkins University Evidence-
based Practice Center shortly after. He
served as a research assistant on the Evidence Review Team.
TG declared no competing interests.
Xuhao Yang, MSPH, is a senior
research coordinator at the Center for
Diversity in Public Health Leadership,
Kennedy Krieger Institute. He also
works at the Johns Hopkins Univer-
sity Evidence-based Practice Center as
a part-time research assistant. He
holds a Bachelor of Science degree in
Global Health from Wuhan Univer-
sity in China and a Master of Science
in Public Health from the Johns Hopkins Bloomberg School of
Public Health. He provides statistical solutions and fulfills data
evaluation needs. He is interested in translating evidence-based
practices into community health services and public health
training programs. He assisted in several systematic review
projects with the Johns Hopkins Bloomberg School of Public
Health team. He supported the GRADE development of a
WHO 2021 Guideline Development project on self-care/online
interventions for sexual and repr oductiv e health among ke y
populations. He collaborated on a systematic review and meta-
analysis project with the Johns Hopkins team to evaluate the
impact of needle and syringe exchange programs on needle-
sharing behaviors and other HIV -related outcomes in low- and
middle-income countries. He also served as a research assistant
on the Evidence R eview Team.
XY declared no competing interests.
biographic and disclosure information www.kidney-international.org
S292
Kidney International (2024) 105 (Suppl 4S), S117–S314

Verna Lazar, MBBS, MPH, is a
research associate in the Department
of International Health at Johns
Hopkins Bloomberg School of Public
Health. She earned her medical de-
gree from St. John’s Medical College,
India, and MPH from Johns Hopkins
University, MD, USA. She has expe-
rience in the conduct of epidemio-
logical studies, locally and internationally. Her major research
interests lie in maternal and child health and health services
research. She served as a graduate research assistant on the
Evidence Review Team.
VL declared no competing interests.
Jeongmin Hana Kim, PharmD,
MSc, is a pharmacist with training in
pharmacoepidemiology, combining
academic training with practical in-
dustry experience in multiple coun-
tries. Hana’s research interests
encompass evidence-based medicine,
literature review, real-world evidence,
drug safety, and effectiveness. She has
a background in clinical research, medical information, and
pharmacovigilance spanning several years in the pharmaceu-
tical industry. She holds a Master of Science degree in Epide-
miology with a concentration in pharmacoepidemiology from
Johns Hopkins Bloomberg School of Public Health. She served
as a graduate research assistant on the Evidence Review Team.
JHK declared no competing interests.
www.kidney-international.org biographic and disclosure information
Kidney International (2024) 105 (Suppl 4S), S117–S314 S293

Acknowledgments
A special debt of gratitude is owed to the KDIGO Co-Chairs,
Morgan Grams and Michel Jadoul, and immediate past Co-
Chair Wolfgang Winkelmayer, for their invaluable oversig ht
throughout the development of this guideline. In particular,
we thank Karen Robinson, Lisa Wilson, Renee Wilson, Dipal
Patel, and the ERT members for their substantial contribution
to the rigorous assessment of the available evidence. We
acknowledge Bertram Kasiske and Marcello Tonelli for their
guidance on strengthening the linkage between the recom-
mendations and evidence base and for striving to improve on
the format to be tter meet the KDIGO aspiration for a “living
guideline” that is consistently kept up to date, and above all,
useful and informative to practicing healthcare providers. We
also would like to acknowledge Debbie Maizels for her vital
contributions to the artwork presented in this guideline.
We are especially grateful to the Work Group members for
their expertise throughout the entire process of literature
review, data extraction, meeting participation, and the critical
writing and editing of the statements and rationale, which
made the publication of this guideline possible. The generous
gift of their time and dedication is greatly appreciated. Finally,
on behalf of the Work Group, we gratefully acknowledge the
careful assessment of the draft guideline by public external
reviewers. The Work Group considered all of the valuable
comments made, and where appropriate, suggested changes
were incorporated into the final publication. The following
individuals provided feedback during the public review of the
draft guideline:
Muhammad Adnan, Baris Afsar, Naseer Ahmed, Naif
Alghamdi, American Society of Onconephrology, Patricia
Abreu, Mabel Aoun, Mustafa Arici, Mariano Arriola, Elisabet
Ars, Iso Asp, AstraZeneca/Alexion, Randa Ataya, Carla Maria
Avesani, George Bakris, Bayer AG, Dennis Begos, Nouha Ben
Mahmoud, An atole Besarab, Sangeeta Bhorade, Jordi Bover,
Rafael Burgos Calderon, Laura Byham-Gray, Tatiana Car-
dona, Marta Carlesso, Alexander Chang, Linh Chi, Kay Weng
Choy, Cather ine Clase, Rolando Claure-Del Granado,
Veronica Coll Brito, Dervla Connaughton, Valerio Coronel,
Kathy Crotts, CSL/Vifor, Adamasco Cupisti, Kader Dag-
hastanli, Neera Dahl, Luca De Nicola, Rogrerio de Paula,
Desiree de Waal, Pierre Delanaye, Luca Di Lullo, Lauren
Dight, Nida Dinçel, Nicole Domanski, Michael Donovan,
Katie Durman, Ogo Egbuna, Nagy Eid, Alicia Elbert, Amal
Eldegheili, Mohamed Elrggal, Magdy Elsharkawy, Ruben
Escalante, Marino Fernandez, Beatriz Fernandez-Fernandez,
Jorge Flores, Vivian Fonseca, Gnidela Fouzia, Nora France-
schini, Liliana Garneata, Michael Germain, Ali Gharavi,
Richard Glassock, Manuel Gorostidi, Carolina Gracia-Iguacel,
GlaxoSmithKline, Mostapha Habib Allah, Meg Hager, Ivory
Harding, Kathy Harvey, Abdelazem Hassan Mohamed Awad,
Thato Hlokwe, Thomas Idorn, Kunitoshi Iseki, Lily Jakulj,
Faical Jarraya, Stuart Jennings, Chandra Mauli Jha, Norman
Jiménez, Swapna Joseph, Shivam Joshi, Natthaphong Juroja-
nanukul, Sanjay Kalra, Nada Kanaan, Deepa Kariyawasam,
Harvey Kaufman, Titi Kazeem, Andrea King, Joshua Kiptoo,
Krzysztof Kiryluk, Nine Knoers, Jennefer Kohler, Manjunath
Kulkarni, Andrew Lazar, Quy Lê, Claudia Fernanda Leiva
Gómez, Edgar Lerma, Laura Lerner, Andrew S. Levey,
Eduardo Lorca Herrera, Racquel Lowe-Jones, Valerie Luyckx,
Bruno Mafrici, Rida Malik, Partha Pratim Mandal, Harold
Manley, El isabet Masso, Anthony Meade, Seceleanu Mirela,
Karine Moreau, Raquib Morshed, Eugen Mota, Michael
Murphy, Devika Nair, Alexandre Neves Gonçalves, Tze Jian
Ng, Lian Ni, Ulf Nyman, Ugochi Onu, Alberto Ortiz, Cem
Oztop, Meyeon Park, Jessie Pavlinac, Saime Paydas, Adriana
Penalba, Graciela Pennacchiotti, Emmanuel Ernesto Perez
Granados, Nuria S. Perez Romano, Vinh Pham Quang, Phaly
Phon, Giorgina Piccoli, Cheryl Rajah, Abdelhamid Hamdy
Ramadan, Elvia Ramíre, Jeannette Rautenbach, Mary- Beth
Roberts, Nicolas Roberto Robles, Jakub Ruszkowski, Bilquees
Saba, Alice Sabatino, Judy Savige, John Sayer, Anja Selig,
Wendy St. Peter, Megan Stoutz, Veronica Torres, Stephanie
Toth-Manikowski, Sri Lekha Tummalapalli, Vertex, Carlos
Villegas, Michelangelo Viscione, Er ic Wallace, Darcy Weide-
mann, B. André Weinstock, Christine White, Katy Wilkens,
and Andrea Zimmermann.
Participation in the public review does not necessarily
constitute endorsement of the content of this report by the
above individuals, or the organizations or institutions they
represent.
acknowledgments www.kidney-international.org
S294
Kidney International (2024) 105 (Suppl 4S), S117–S314

References
1. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO
2012 clinical practice guideline for the evaluation and management of
chronic kidney disease. Kidney Int Suppl. 2012;3:S1–S150.
2. National Kidney Foundation. K/DOQI clinical practice guidelines for
chronic kidney disease: evaluation, classi fication, and stratification. Am
J Kidney Dis. 2002;39(suppl 1):S1–S266.
3. Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and
prognosis of chronic kidney disease: a KDIGO Controversies
Conference report. Kidney Int. 2011;80:17–28.
4. Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der
Velde M, et al. Association of estimated glomerular filtration rate and
albuminuria with all-cause and cardiovascular mortality in general
population cohorts: a collaborative meta-analysi s. Lancet . 2010;375:
2073–2081.
5. Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated
GFR and higher albuminuria are associated with adverse kidney
outcomes. A collaborative meta-analysis of general and high-risk
population cohorts. Kidney Int. 2011;80:93–104.
6. Grams ME, Sang Y, Ballew SH, et al. Predicting timing of clinical
outcomes in patients with chronic kidney disease and severely
decreased glomerular filtration rate. Kidney Int. 2018;93:1442–1451.
7. van der Velde M, Matsushita K, Coresh J, et al. Lower estimated
glomerular filtration rate and higher albuminuria are associated with
all-cause and cardiovascular mortality. A collaborative meta-analysis of
high-risk population cohorts. Kidney Int. 2011;79:1341–1352.
8. Ferguson T, Ravani P, Sood MM, et al. Development and external
validation of a machine learning model for progression of CKD. Kidney
Int Rep. 2022;7:1772–1781.
9. Tangri N, Grams ME, Levey AS, et al. Multinational assessment of
accuracy of equations for predicting risk of kidney failure: a meta-
analysis. JAMA. 2016;315:164–174.
10. Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression
of chronic kidney disease to kidney failure. JAMA. 2011;305:1553–1559.
11. Delanaye P, Jager KJ, Bokenkamp A, et al. CKD: a call for an age-adapted
definition. J Am Soc Nephrol. 2019;30:1785–1805.
12.
Grams ME, Coresh J, Matsushita K, et al. Estimated glomerular filtration
rate,
albuminuria, and adverse outcomes: an individual-participant
data meta-analysis. JAMA. 2023;330:1266–1277.
13. Wetzels JF, Kiemeney LA, Swinkels DW, et al. Age- and gender-specific
reference values of estimated GFR in Caucasians: the Nijmegen
Biomedical Study. Kidney Int. 2007;72:632–637.
14. Lindeman RD, Tobin J, Shock NW. Longitudinal studies on the rate of
decline in renal function with age. J Am Geriatr Soc . 1985;33:278–285.
15. Rowe JW, Andres R, Tobin JD. Letter: age-adjusted standards for
creatinine clearance. Ann Intern Med. 1976;84:567–569.
16. Komenda P, Ferguson TW, Macdonald K, et al. Cost-effectiveness of
primary screening for CKD: a systematic review. Am J Kidney Dis.
2014;63:789–797.
17. Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early
identification and intervention of chronic kidney disease: conclusions
from a Kidney Disease: Improving Global Outcomes (KDIGO)
Controversies Conference. Kidney Int. 2021;99:34–47.
18. Venuthurupalli SK, Hoy WE, Healy HG, et al. CKD screening and
surveillance in Australia: past, present, and future. Kidney Int Rep.
2018;3:36–46.
19. Kidney Disease: Improving Global Outcomes Lipids Work Group. KDIGO
Clinical Practice Guideline for Lipid Management in Chronic Kidney
Disease. Kidney Int Suppl. 2013;3:S1– S305.
20. Kidney Disease: Improving Global Outcomes CKD-MBD Work Group.
KDIGO 2017 clinical practice guideline update for the diagnosis,
evaluation, prevention, and treatment of chronic kidney disease-
mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2017;7:1–59.
21. Kidney Disease: Improving Global Outcomes Blood Pressure Work
Group. KDIGO 2021 clinical practice guideline for the management of
blood pressure in chronic kidney disease. Kidney Int. 2021;99(3S):S1–S87.
22. Kidney Disease: Improving Global Outcomes Glomerular Diseases Work
Group. KDIGO 2021 clinical practice guideline for the management of
glomerular diseases. Kidney Int. 2021;100(4S):S1–S276.
23. Kidney Disease: Improving Global Outcomes Diabetes Work Group.
KDIGO 2022 clinical practice guideline for diabetes management in
chronic kidney disease. Kidney Int. 2022;102(5S):S1–S127.
24. Global Burden of Disease 2019: GBD cause and risk summaries chronic
kidney disease. Lancet. 2020;396:S152–S153.
25. GBD
Chronic Kidney Disease Collaboration. Global, regional, and
national burden of chronic kidney disease, 1990-2017: a systematic
analysis for the Global Burden of Disease Study 2017. Lancet.
2020;395:709–733.
26. Levin A, Okpechi IG, Caskey FJ, et al. Perspectives on early detection of
chronic kidney disease: the facts, the questions, and a proposed
framework for 2023 and beyond. Kidney Int. 2023;103:1004–1008.
27. Cusick MM, Tisdale RL, Chertow GM, et al. Population-wide screening for
chronic kidney disease: a cost-effectiveness analysis. Ann Intern Med.
2023;176:788–797.
28. Lameire NH, Levin A, Kellum JA, et al. Harmonizing acute and chronic
kidney disease definition and classification: report of a Kidney Disease:
Improving Global Outcomes (KDIGO) Consensus Conference. Kidney
Int. 2021;100:516– 526.
29. de Boer IH, Khunti K, Sadusky T, et al. Diabetes management in chronic
kidney disease: a consensus report by the American Diabetes
Association (ADA) and Kidney Disease: Improving Global Outcomes
(KDIGO). Kidney Int. 2022;102:974–989.
30. Nelson RG, Grams ME, Ballew SH, et al. Development of risk prediction
equations for incident chronic kidney disease. JAMA. 2019;322:2104–
2114.
31. Bello AK, Levin A, Tonelli M, et al. Assessment of Global Kidney Health
Care Status. JAMA. 2017;317:1864–1881.
32. Stanifer JW, Von Isenburg M, Chertow GM, et al. Chronic kidney disease
care models in low- and middle-income countries: a systematic review.
BMJ Glob Health. 2018;3:e000728.
33. Myers GL, Miller WG. The International Consortium for Harmonization of
Clinical Laboratory Results (ICHCLR)—a pathway for harmonization.
EJIFCC. 2016;27:30–36.
34. Myers GL, Miller WG. The roadmap for harmonization: status of the
International Consortium for Harmonization of Clinical Laboratory
Results. Clin Chem Lab Med. 2018;56:1667–1672.
35. Warady BA, Chadha V. Chronic kidney disease in children: the global
perspective. Pediatr Nephrol. 2007;22:1999–2009.
36. Furth SL, Abraham AG, Jerry-Fluker J, et al. Metabolic abnormalities,
cardiovascular disease risk factors, and GFR decline in children with
chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2132–2140.
37. Canadian Institutes of Health Research. What is gender? What is sex?.
Accessed May 29, 2023. https://cihr-irsc.gc.ca/e/48642.html
38. Carrero JJ, Hecking M, Chesnaye NC, et al. Sex and gender disparities in
the epidemiology and outcomes of chronic kidney disease. Nat Rev
Nephrol. 2018;14:151–
164.
39. Bairey
Merz CN, Dember LM, Ingelfinger JR, et al. Sex and the kidneys:
current unders tanding and research opportunities. Nat Rev Nephrol.
2019;15:776–783.
40. Cobo G, Hecking M, Port FK, et al. Sex and gender differences in chronic
kidney disease: progression to end-stage renal disease and
haemodialysis. Clin Sci (Lond). 2016;130:1147–1163.
41. Neugarten J, Acharya A, Silbiger SR. Effect of gender on the progression
of nondiabetic renal disease: a meta-analysis. J Am Soc Nephrol. 2000;11:
319–329.
42. Swartling O, Rydell H, Stendahl M, et al. CKD progression and mortality
among men and women: a nationwide study in Sweden. Am J Kidney
Dis. 2021;78:190–199.e191.
43. Jafar TH, Schmid CH, Stark PC, et al. The rate of progression of
renal disease may not be slower in women compared with men:
a patient-level meta-analysis. Nephrol Dial Transplant. 2003;18:2047–
2053.
44. Nitsch D, Grams M, Sang Y, et al. Associations of estimated glomerular
filtration rate and albuminuria with mortality and renal failure by sex:
a meta-analysis. BMJ. 2013;346:f324.
45. Ahmed SB. The importance of sex and gender in basic and clinical
research. Nat Rev Nephrol. 2024;20:2–3.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S295

46. Bots SH, Schreuder MM, Roeters van Lennep JE, et al. Sex differences in
reported adverse drug reactions to angiotensin-converting enzyme
inhibitors. JAMA. Netw Open. 2022;5:e228224.
47. Garcia GG, Iyengar A, Kaze F, et al. Sex and gender differences in chronic
kidney disease and access to care around the globe. Semin Nephrol.
2022;42:101–113.
48. Swartling O, Yang Y, Clase CM, et al. Sex differences in the recognition,
monitoring, and management of CKD in health care: an observational
cohort study. J Am Soc Nephrol. 2022;33:1903–1914.
49. Ahmed SB, Beach LB, Safer JD, et al. Considerations in the care of
transgender person s. Nat Rev Nephrol. 2023;19:360–365.
50. Dumanski SM, Eckersten D, Piccoli GB. Reproductive health in chronic
kidney disease: the implications of sex and gender. Semin Nephrol.
2022;42:142–152.
51. Wiles KS, Nelson-Piercy C, Bramham K. Reproductive health and
pregnancy in women with chronic kidney disease. Nat Rev Nephrol.
2018;14:165–184.
52. Barrett PM, McCarthy FP, Kublickiene K, et al. Adverse pregnancy
outcomes and long-term maternal kidney disease: a systematic review
and meta-analysis. JAMA Netw Open. 2020;3:e1920964.
53. Al Khalaf S, Bodunde E, Maher GM, et al. Chronic kidney disease and
adverse pregnancy outcomes: a systematic review and meta-analysis.
Am J Obstet Gynecol. 2022;226:656–670.e632.
54. Wiles K, Chappell L, Clark K, et al. Clinical practice guideline on
pregnancy and renal disease. BMC Nephrol. 2019;20:401.
55. Delanaye P, Glassock RJ, Pottel H, et al. An age-calibrated definition of
chronic kidney disease: rationale and benefits. Clin Biochem Rev.
2016;37:17–26.
56. Palmer Alves T, Lewis J. Racial differences in chronic kidney disease
(CKD) and end-stage renal disease (ESRD) in the United States: a social
and economic dilemma. Clin Nephrol. 2010;74(suppl 1):S72–S77.
57. Li PK, Garcia-Garcia G, Lui SF, et al. Kidney health for everyone
everywhere-from prevention to detection and equitable access to
care. Kidney Int. 2020;97:226–232.
58. Charles C, Gafni A, Whelan T. Shared decision-making in the medical
encounter: what does it mean? (or it takes at least two to tango). Soc
Sci Med. 1997;44:681–692.
59. Hoffmann TC, Montori VM, Del Mar C. The connection between
evidence-based medicine and shared decision making. JAMA.
2014;312:1295
–1296.
60. Naik
AD, Kallen MA, Walder A, et al. Improving hypertension control in
diabetes mellitus: the effects of collaborative and proactive health
communication. Circulation. 2008;117:1361–1368.
61. Andermann A, Blancquaert I, Beauchamp S, et al. Revisiting Wilson and
Jungner in the genomic age: a review of screening criteria over the past
40 years. Bull World Health Organ. 2008;86:317–319.
62. Levin A, Stevens PE. Early detection of CKD: the benefits, limitations and
effects on prognosis. Nat Rev Nephrol. 2011;7:446–457.
63. Wilson JMG, Jungner G. Principles and Practice of Screening for Disease.
World Health Organization; 1968.
64. Hemmelgarn BR, Pannu N, Ahmed SB, et al. Determining the research
priorities for patients with chronic kidney disease not on dialysis.
Nephrol Dial Transplant. 2017;32:847–854.
65. Um YJ, Chang Y, Kim Y, et al. Risk of CKD following detection of
microscopic hematuria: a retrospective cohort study. Am J Kidney Dis.
2023;81:425–433.e421.
66. Barocas DA, Boorjian SA, Alvarez RD, et al. Microhematuria: AUA/SUFU
guideline. J Urol. 2020;204:778–786.
67. Ingelfinger JR. Hematuria in adults. N Engl J Med. 2021;385:153–163.
68. Crump C, Sundquist J, Winkleby MA, et al. Preterm birth and risk of
chronic kidney disease from childhood into mid-adulthood: national
cohort study. BMJ. 2019;365:l1346.
69. Luyckx VA, Brenner BM. Birth weight, malnutrition and kidney-associated
outcomes—aglobalconcern.Nat Rev Nephrol. 2015;11:135–149.
70. Luyckx VA, Perico N, Somaschini M, et al. A developmental approach to
the prevention of hypertension and kidney disease: a report from the
Low Birth Weight and Nephron Number Work ing Group. Lancet.
2017;390:424–428.
71. Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million
adolescents and risk for end-stage renal disease. Arch Intern Med.
2012;172:1644–1650.
72. Chaturvedi S, Ng KH, Mammen C. The path to chronic kidney disease
following acute kidney injury: a neonatal perspective. Pediatr Nephrol.
2017;32:227–241.
73. Allen AM, Kim WR, Larson JJ, et al. Serum cystatin C as an indicator of
renal function and mortality in liver transplant recipients.
Transplantation.
2015;99:1431–1435.
74. Bhasin B, Lau B, Atta MG, et al. HIV viremia and T-cell activation
differentially affect the performance of glomerular filtration rate
equations based on creatinine and cystatin C. PLoS One. 2014;8:e82028.
75. Björk J, Grubb A, Gudnason V, et al. Comparison of glomerular filtration
rate estimating equations derived from creatinine and cystatin C:
validation in the Age, Gene/Environment Susceptibility-Reykjavik
elderly cohort. Nephrol Dial Transplant. 2017;33:1380–1388.
76. Bluhme E, Malenicka S, Fischler B, et al. Comparison of cystatin C,
creatinine, and iohexol clearance in pediatric liver transplantat ion-a
retrospective cohort study. Pediatr Transplant. 2021;25:e13993.
77. Bukabau JB, Sumaili EK, Cavalier E, et al. Performance of glomerular
filtration rate estimation equations in Congolese healthy adults: the
inopportunity of the ethnic correction. PLoS One. 2018;13:e0193384.
78. Chen N, Shi H, Zhang L, et al. GFR estimation using a panel of filtration
markers in Shanghai and Beijing. Kidney Med. 2020;2:172–180.
79. De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus
cystatin C-based equations in assessing the renal function of
candidates for liver transplantation with cirrhosis. Hepatology. 2014;59:
1522–1531.
80. Fan L, Inker LA, Rossert J, et al. Glomerular filtration rate estimation using
cystatin C alone or combined with creatinine as a confirmatory test.
Nephrol Dial Transplant. 2014;29:1195–1203.
81. Fan L, Levey AS, Gudnason V, et al. Comparing GFR estimating equations
using cystatin C and creatinine in elderly individuals. J Am Soc Nephrol.
2014;26:1982–1989.
82. Fu EL, Levey AS, Coresh J, et al. Accuracy of GFR estimating equations in
patients with discordances between creatinine and cystatin C-based
estimations. J Am Soc Nephrol. 2023;34:1241–1251.
83. Horio M, Imai E, Yasuda Y, et al. GFR estimation using standardized
serum cystatin C in Japan. Am J Kidney Dis. 2012;61:197–203.
84. Inker LA, Levey AS, Tighiouart H, et al. Performance of glomerular
filtration rate estimating equations in a community-based sample of
Blacks and Whites: the multiethnic study of atherosclerosis. Nephrol
Dial Transplant. 2017;33:417–425.
85. Lennartz CS, Pickering JW, Seiler-Mussler S, et al. External validation of
the kidney failure risk equation and re-calibration with addition of
ultrasound parameters.
Clin J Am Soc Nephrol.
2016;11:609–615.
86. Liu X, Ma H, Huang H, et al. Is the Chronic Kidney Disease Epidemiology
Collaboration creatinine-cystatin C equation useful for glomerular
filtration rate estimation in the elderly? Clinical Interv Aging. 2013;8:
1387–1391.
87. Lopes MB, Araújo LQ, Passos MT, et al. Estimation of glomerular fi ltration
rate from serum creatinine and cystatin C in octogenarians and
nonagenarians. BMC Nephrol. 2013;14:265.
88. Machado JD, Camargo EG, Boff R, et al. Combined creatinine-cystatin C
CKD-EPI equation significantly underestimates measured glomerular
filtration rate in people with type 2 diabetes mellitus. Clin Biochem.
2018;53:43–48.
89. Marks A, Fluck N, Prescott GJ, et al. Looking to the future: predicting
renal replacement outcomes in a large community cohort with
chronic kidney disease. Nephrol Dial Transplant. 2015;30:1507–1517.
90. Medina Arnaudo GI. [Evaluation of equations using cystatin C for
estimation of the glomerular filtration rate in healthy adult population
of candidates for kidney donors]. Rev Fac Cien Med Univ Nac Cordoba.
2018;74:243–250 [in Spanish].
91. Pottel H, Björk J, Rule AD, et al. Cystatin C-based equation to estimate
GFR without the inclusion of race and sex. N Engl J Med. 2023;388:
333–343.
92. Schaeffner ES, Ebert N, Delanaye P, et al. Two novel equations to
estimate kidney function in persons aged 70 years or older. Ann Intern
Med. 2012;157:471–481.
93. Wang Y, Levey AS, Inker LA, et al. Performance and determinants of
serum creatinine and cystatin C-based GFR estimating equations in
South Asians. Kidney Int Rep. 2021;6:962–975.
94. Wang Y, Nguyen F, Allen JC, et al. Validation of the kidney failure risk
equation for end-stage kidney disease in Southeast Asia. BMC Nephrol.
2019;20:451.
95. Werner K, Pihlsgård M, Elmståhl S, et al. Combining cystatin C and
creatinine yields a reliable glomerular filtration rate estimation in older
adults in contrast to
b
-trace protein and
b
2-microglobulin. Nephron.
2017;137:29–37.
references www.kidney-international.org
S296
Kidney International (2024) 105 (Suppl 4S), S117–S314

96. Whitlock RH, Chartier M, Komenda P, et al. Validation of the kidney
failure risk equation in Manitoba. Can J Kidney Health Dis. 2017;4:
2054358117705372.
97. Kidney Disease: Improving Global Outcomes AKI Work Group. KDIGO
clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–
138.
98. Leung N, Bridoux F, Batuman V, et al. The evaluation of monoclonal
gammopathy of renal significance: a consensus report of the
International Kidney and Monoclonal Gammopathy Research Group.
Nat Rev Nephrol. 2019;15:45–59.
99. Knoers N, Antignac C, Bergmann C, et al. Genetic testing in the diagnosis
of chronic kidney disease: recommendations for clinical practice. Nephrol
Dial Transplant . 2022;37:239–254.
100. KDIGO Conference Participants. Genetics in chronic kidney disease:
conclusions from a Kidney Disease: Improving Global Outcomes
(KDIGO) Controversies Conference. Kidney Int. 2022;101:1126– 1141.
101. Schrezenmeier E, Kremerskothen E, Halleck F, et al. The underestimated
burden of monogenic kidney disease in adults waitlisted for kidney
transplantation. Genet Med. 2021;23:1219–1224.
102. Groopman EE, Marasa M, Cameron-Christie S, et al. Diagnostic utility of
exome sequencing for kidney disease. N Engl J Med. 2019;380:142–151.
103. Bleyer AJ, Westemeyer M, Xie J, et al. Genetic etiologies for chronic
kidney disease revealed through next-generation renal gene panel.
Am J Nephrol. 2022;53:297–306.
104. Jayasinghe K, Stark Z, Kerr PG, et al. Clinical impact of genomic testing in
patients with suspected monogenic kidney disease. Genet Med. 2021;23:
183–191.
105. Cocchi E, Nestor JG, Ghara vi AG. Clinical genetic screening in adult
patients with kidney disease. Clin J Am Soc Nephrol. 2020;15:1497–1510.
106. Altindal M, Yildirim T, Turkmen E, et al. Safety of percutaneous
ultrasound-guided kidney biopsy in patients with AA amyloidosis.
Nephron. 2015;131:17–22.
107. Chen TK, Estrella MM, Fine DM. Predictors of kidney biopsy complication
among patients with systemic lupus erythematosus. Lupus. 2012;21:848–
854.
108. Dong L, Li J, Zhao M, et al. Application of B-ultrasound information
image in renal puncture biopsy treatment and nursing. Pak J Med Sci.
2021;37:1564–1568.
109. Eiro M, Katoh T, Watanabe T. Risk factors for bleeding complications in
percutaneous renal biopsy. Clin Exp Nephrol. 2005;9:40–45.
110.
Gimenez LF, Micali S, Chen RN, et al. Laparoscopic renal biopsy. Kidne
y
Int. 1998;54:525 – 529.
111. Jordan N, Chaib A, Sangle S, et al. Association of thrombotic
microangiopathy and intimal hyperplasia with bleeding post-renal
biopsy in antiphospholipid antibody-positive patients. Arthritis Care Res
(Hoboken). 2014;66:725–731.
112. Joseph AJ, Compton SP, Holmes LH, et al. Utility of percutaneous
renal biopsy in chronic kidney disease. Nephrology (Carlton). 2010;15:
544–548.
113. Malvar A, Alberton V, Lococo B, et al. Kidney biopsy-based management
of maintenance immunosuppression is safe and may ameliorate flare
rate in lupus nephritis. Kidney Int. 2020;97:156–162.
114. Manno C, Bonifati C, Torres DD, et al. Desmopressin acetate in
percutaneous ultrasound-guided kidney biopsy: a randomized
controlled trial. Am J Kidney Dis. 2011;57:850–855.
115. Manno C, Strippoli GF, Arnesano L, et al. Predictors of bleeding
complications in percutaneous ultrasound-guided renal biopsy. Kidney
Int. 2004;66:1570–1577.
116. Mejía-Vilet JM, Márquez-Martínez MA, Cordova-Sanchez BM, et al. Simple
risk score for prediction of haemorrhagic complications after a
percutaneous renal biopsy. Nephrology (Carlton). 2018;23:523–529.
117. Moulin B, Dhib M, Sommervogel C, et al. [Value of renal biopsy in the
elderly. 32 cases]. Presse Med. 1991;20:1881–1885 [in French].
118. Nadium WK, Abdelwahab HH, Ibrahim MA, et al. Histological pattern of
primary glomerular diseases among adult Sudanese patients: a single
center experience. Indian J Nephrol. 2013;23:176–179.
119. Pan CF, Chen YC, Chen HS, et al. Renal biopsy in the elderly: Analysis of
ninety-four cases in a single center, MMH. J Intern Med Taiwan. 2003;14:
69–76.
120. Restrick LJ, Blomley MJ, Drayson RA, et al. Percutaneous renal biopsy in
the district general hospital. J R Coll Physicians Lond. 1993;27:247–251.
121. Roccatello D, Sciascia S, Rossi D, et al. Safety of outpatient percutaneous
native renal biopsy in systemic autoimmune diseases: results from a
monocentric cohort. Lupus. 2018;27:1393–1394.
122. Sarabu N, Maddukuri G, Munikrishnappa D, et al. Safety and efficacy of
transjugular renal biopsy performed by interventional nephrologists.
Semin Dial. 2011;24:343–348.
123. Sobh
M, Moustafa F, Ghoniem M. Value of renal biopsy in chronic renal
failure. Int Urol Nephrol. 1988;20:77 – 83.
124. Tøndel C, Vikse BE, Bostad L, et al. Safety and complications of
percutaneous kidney biopsies in 715 children and 8573 adults in
Norway 1988-2010. Clin J Am Soc Nephrol. 2012;7:1591 – 1597.
125. Tsapenko M, El-Zoghby ZM, Sethi S. Renal histological lesions and
outcome in liver transplant recipients. Clin Transplant. 2012;26:E48–E54.
126. Zhang PP, Ge YC, Li SJ, et al. Renal biopsy in type 2 diabetes: timing of
complications and evaluating of safety in Chinese patients. Nephrology
(Carlton). 2011;16:100–105.
127. Delanaye P, Cavalier E, Radermecker RP, et al. Cystatin C or creatinine for
detection of stage 3 chronic kidney disease in anorexia nervosa. Nephron
Clin Pract. 2008;110:c158–c163.
128. Thurlow JS, Abbott KC, Linberg A, et al. SCr and SCysC concentrations
before and after traumatic amputation in male soldiers: a case-control
study. Am J Kidney Dis. 2014;63:167–170.
129. Knight EL, Verhave JC, Spiegelman D, et al. Factors influencing serum
cystatin C levels other than renal function and the impact on renal
function measurement. Kidney Int. 2004;65:1416–1421.
130. Stevens LA, Schmid CH, Greene T, et al. Factors other than glomerular
filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660.
131. Wang Y, Adingwupu OM, Shlipak MG, et al. Discrepancies between
creatinine and cystatin C-based eGFR: interpretation according to
performance compared to measured GFR. Kidney Med. 2023;5:100710.
132. Hingorani S, Pao E, Schoch G, et al. Estimating GFR in adult patients with
hematopoietic cell transplant: comparison of estimating equations with
an iohexol reference standard. Clin J Am Soc Nephrol. 2015;10:601–610.
133. Matsuoka D, Hirabayashi K, Murase T, et al. Assessment of kidney
function using inulin-based and estimated glomerular filtration rates
before and after allogeneic hematopoietic stem cell transplantation in
pediatric patients. Pediatr Blood Cancer. 2020;67:e28733.
134. Shibata K, Yasuda Y, Kobayashi R, et al. Renal function evaluation in
patients with cancer who were scheduled to receive carboplatin or S-
1. Clin Exp Nephrol. 2015;19:1107–1113.
135. Costa E, Silva VT, Gil LA, Caires RA, et al. Assessment of estimated
glomerular filtration rate in a cohort of 1200 cancer patients using
serum creatinine and cystatin C. J Am Soc Nephrol. 2020;31:11.
136. Costa E, Silva VT, Gil LA Jr, Inker LA, et al. A prospective cross-sectional
study estimated glomerular filtration rate from creatinine and cystatin C
in adults with solid tumors. Kidney
Int. 2022;101:607–614.
137. Kervella D, Lemoine S, Sens F, et al. Cystatin C versus creatinine for GFR
estimation in CKD due to heart failure. Am J Kidney Dis. 2017;69:321–323.
138. Swolinsky JS, Nerger NP, Leistner DM, et al. Serum creatinine and
cystatin C-based estimates of glomerular filtration rate are misleading
in acute heart failure. ESC Heart Fail. 2021;8:3070–3081.
139. Torre A, Aguirre-Valadez JM, Arreola-Guerra JM, et al. Creatinine versus
cystatin C for estimating GFR in patients with liver cirrhosis. Am J
Kidney Dis. 2015;67:342–344.
140. Stammler F, Derain-Dubourg L, Lemoine S, et al. Impact of race-
independent equations on estimating glomerular filtration rate for the
assessment of kidney dysfunction in liver disease. BMC Nephrol.
2023;24:83.
141. Aldenbratt A, Lindberg C, Johannesson E, et al. Estimation of kidney
function in patients with primary neuromuscular diseases: is serum
cystatin C a better marker of kidney function than creatinine? J
Nephrol. 2022;35:493–503.
142. Adingwupu OM, Barbosa ER, Palevsky PM, et al. Cystatin C as a GFR
estimation marker in acute and chronic illness: a systematic review.
Kidney Med. 2023;5:100727.
143. Hemmelgarn BR, Zhang J, Manns BJ, et al. Nephrology visits and health
care resource use before and after reporting estimated glomerular
filtration rate. JAMA. 2010;303:1151–1158.
144. Jain AK, McLeod I, Huo C, et al. When laboratories report estimated
glomerular filtration rates in addition to serum creatinines, nephrology
consults increase. Kidney Int. 2009;76:318–323.
145. Noble E, Johnson DW, Gray N, et al. The impact of automated eGFR
reporting and education on nephrology service referrals. Nephrol Dial
Transplant. 2008;23:3845–3850.
146. Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants
in the Chron ic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis.
2012;60:250–261.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S297

147. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-
based equations to estimate GFR without race. N Engl J Med.
2021;385:1737–1749.
148. Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration
rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29.
149. Pottel H, Delanaye P, Schaeffner E, et al. Estimating glomerular filtration
rate for the full age spectrum from serum creatinine and cystatin C.
Nephrol Dial Transplant. 2017;32:497–507.
150. Gunawardhana L, Becker MA, Whelton A, et al. Efficacy and safety of
febuxostat extended release and immediate release in patients with
gout and moderate renal impairment: phase II placebo-controlled
study. Arthritis Res Ther. 2018;20:99.
151. Teo BW, Koh YY, Toh QC, et al. Performance of the CKD-EPI creatinine-
cystatin C glomerular filtration rate estimation equations in a
multiethnic Asian population. Singapore Med J. 2015;55:656–659.
152. Teo BW, Xu H, Wang D, et al. Estimating glomerular filtration rates by use
of both cystatin C and standardized serum creatinine avoids ethnicity
coefficients in Asian patients with chronic kidney disease. Clin Chem.
2011;58:450–457.
153. Teo BW, Zhang L, Guh JY, et al. Glomerular filtration rates in Asians. Adv
Chronic Kidney Dis. 2018;25:41–48.
154. Zhang M, Chen Y, Tang L, et al. Applicability of chronic kidney disease
epidemiology collaboration equations in a Chinese population.
Nephrol Dial Transplant. 2014;29:580–586.
155. Gagneux-Brunon A, Delanaye P, Maillard N, et al. Performance of
creatinine and cystatin C-based glomerular filtration rate estimating
equations in a European HIV-positive cohort. AIDS. 2013;27:1573–1581.
156. Inker LA, Wyatt C, Cre amer R, et al. Performance of creatinine and
cystatin C GFR estimating equations in an HIV-positive population on
antiretrovirals. J Acquir Immune Defic Syndr. 2012;61:302–309.
157. Lucas GM, Atta MG, Zook K, et al. Cross-sectional and longitudinal
performance of creatinine- and cystatin C-based estimating equations
relative to exogenously measured glomerular filtration rate in HIV-
positive and HIV-negative persons. J Acquir Immune Defic Syndr.
2020;85:e58–
e66.
158. Delanaye
P, Cavalier E, Morel J, et al. Detection of decreased glomerular
filtration rate in intensive care units: serum cystatin C versus serum
creatinine. BMC Nephrol. 2014;15:9.
159. Carlier M, Dumoulin A, Janssen A, et al. Comparison of different
equations to assess glomerular filtration in critically ill patients.
Intensive Care Med. 2015;41:427–435.
160. Sangla F, Marti PE, Verissimo T, et al. Measured and estimated
glomerular filtration rate in the ICU: a prospective study. Crit Care Med.
2020;48:e1232–e1241.
161. Wagner D, Kniepeiss D, Stiegler P, et al. The assessment of GFR after
orthotopic liver transplantation using cystatin C and creatinine-based
equations. Transpl Int. 2012;25:527–536.
162. Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency
results of the BIRMA study. Br J Cancer. 2010;103:1815–1821.
163. Launay-Vacher V, Janus N, Deray G. Renal insufficiency and cancer
treatments. ESMO Open. 2016;1:e000091.
164. Na SY, Sung JY, Chang JH, et al. Chronic kidney disease in cancer
patients: an independent predictor of cancer-specific mortality. Am J
Nephrol. 2011;33:121–130.
165. Rosner MH, Jhaveri KD, McMahon BA, et al. Onconephrology: the
intersections between the kidney and cancer. CA Cancer J Clin.
2021;71:47–77.
166. Soveri I, Berg UB, Bjork J, et al. Measuring GFR: a systematic review. Am J
Kidney Dis. 2014;64:411–424.
167. White CA, Akbari A, Allen C, et al. Simultaneous glomerular filtration rate
determination using inulin, iohexol, and
99m
Tc-DTPA demonstrates the
need for customized measurement protocols. Kidney Int. 2021;99:957–
966.
168. Xie P, Huang JM, Liu XM, et al. (99m)Tc-DTPA renal dynamic imaging
method may be unsuitable to be used as the reference method in
investigating the validity of CDK-EPI equation for determining
glomerular filtration rate. PLoS One. 2013;8:e62328.
169. Rowe C, Sitch AJ, Barratt J, et al. Biological variation of measured and
estimated glomerular filtration rate in patients with chronic kidney
disease. Kidney Int. 2019;96:429–435.
170. Delanaye P, Ebert N, Melsom T, et al. Iohexol plasma clearance for
measuring glomerular filtration rate in clinical practice and research: a
review. Part 1: how to measure glomerular filtration rate with iohexol?
Clin Kidney J. 2016;9:682–699.
171. Kwong YT, Stevens LA, Selvin E, et al. Imprecision of urinary iothalamate
clearance as a gold-standard measur e of GFR decreases the diagnostic
accuracy of kidney function estimating equations. Am J Kidney Dis.
2010;56:39–49.
172. Levey AS, Greene T, Schluchter MD, et al. Glomerular filtration rate
measurements in clinical trials. Modification of Diet in Renal Disease
Study Group and the Diabetes Control and Complications Trial
Research Group. J Am Soc Nephrol. 1993;4:1159–1171.
173. Perrone RD, Steinman TI, Beck GJ, et al. Utility of radioisotopic filtration
markers in chronic renal insufficiency: simultaneous comparison of 125I-
iothalamate, 169Yb-DTPA, 99mTc-DTPA, and inulin. The Modification of
Diet in Renal Disease Study. Am J Kidney Dis. 1990;16:224–235.
174. Inker LA, Levey AS. Knowing your GFR-when is the number not (exactly)
the number? Kidney Int. 2019;96:280–282.
175. Shlipak MG, Inker LA, Coresh J. Serum cystatin C for estimation of GFR.
JAMA. 2022;328:883– 884.
176. Chang AR, Zafar W, Grams ME. Kidney function in obesity-challenges in
indexing and estimation. Adv Chronic Kidney Dis. 2018;25:31–40.
177. Foster MC, Levey AS, Inker LA, et al. Non-GFR determinants of low-
molecular-weight serum protein filtration markers in the elderly: AGES-
kidney and MESA-kidney. Am J Kidney Dis. 2017;70:406–414.
178. Inker
LA, Titan S. Measurement and estimation of GFR for use in clinical
practice: core curriculum 2021. Am J Kidney Dis. 2021;78:736–749.
179. Jayagopal V, Keevil BG, Atkin SL, et al. Paradoxical changes in cystatin C
and serum creatinine in patients with hypo- and hyperthyroidism. Clin
Chem. 2003;49:680 – 681.
180. Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public
health. Am J Kidney Dis. 2014;63:820–834.
181. Liu X, Foster MC, Tighiouart H, et al. Non-GFR determinants of low-
molecular-weight serum protein filtration markers in CKD. Am J Kidney
Dis. 2016;68:892–900.
182. Melsom T, Fuskevag OM, Mathisen UD, et al. Estimated GFR is biased by
non-traditional cardiovascular risk factors. Am J Nephrol. 2015;41:7–15.
183. Schei J, Stefansson VT, Mathisen UD, et al. Residual associations of
inflammatory markers with eGFR after accounting for measured GFR
in a community-based cohort without CKD. Clin J Am Soc Nephrol.
2016;11:280–286.
184. Sjostrom P, Tidman M, Jones I. Determination of the production rate and
non-renal clearance of cystatin C and estimation of the glomerular
filtration rate from the serum concentration of cystatin C in humans.
Scand J Clin Lab Invest. 2005;65:111–124.
185. Xin C, Xie J, Fan H, et al. Association between serum cystatin C and
thyroid diseases: a systematic review and meta-analysis. Front
Endocrinol (Lausanne). 2021;12:766516.
186. Agarwal R, Bills JE, Yigazu PM, et al. Assessment of iothalamate plasma
clearance: duration of study affects quality of GFR. Clin J Am Soc
Nephrol. 2009;4:77–85.
187. Shah KF, Stevens PE, Lamb EJ. The influence of a cooked-fish meal
on estimated glomerular filtration rate. Ann Clin Biochem. 2020;57:182–
185.
188. Inker LA, Levey AS, Coresh J. Estimated glomerular filtration rate from a
panel of filtration markers-hope for increased accuracy beyond
measured glomerular filtration rate? Adv Chronic Kidney Dis. 2018;25:
67–75.
189.
Potok OA, Rifkin DE, Ix JH, et al. Estimated GFR accuracy when cystatin C-
and
creatinine-based estimates are discrepant in older adults. Kidney
Med. 2023;5:100628.
190. Farrington DK, Surapaneni A, Matsushita K, et al. Discrepancies between
cystatin C-based and creatinine-based eGFR. Clin J Am Soc Nephrol.
2023;18:1143–1152.
191. Coresh J, Toto RD, Kirk KA, et al. Creatinine clearance as a measure of
GFR in screenees for the African-American study of kidney disease
and hypertension pilot study. Am J Kidney Dis. 1998;32:32–42.
192. Krupka E, Curtis S, Ferguson T, et al. The effect of gender-affirming
hormone therapy on measures of kidney function: a systematic review
and meta-analysis. Clin J Am Soc Nephrol. 2022;17: 1305 – 1315.
193. van Eeghen SA, Wiepjes CM, T’Sjoen G, et al. Cystatin C-based eGFR
changes during gender-affirming hormone therapy in transgender
individuals. Clin J Am Soc Nephrol. 2023;18:1545–1554.
194. Pierre C, Marzinke M, Ahmed SB, et al. AACC/NKF guidance document on
improving equity in chronic kidney disease care. J Appl Lab Med. 2023;8:
789–816.
195. Ng DK, Furth SL, Warady BA, et al. Self-repo rted race, serum creatinine,
cystatin C, and GFR in children and young adults with pediatric kidney
references www.kidney-international.org
S298
Kidney International (2024) 105 (Suppl 4S), S117–S314

diseases: a report from the Chronic Kidney Disease in Children (CKiD)
study. Am J Kidney Dis. 2022;80:174–185.e1.
196. Luna-Zaizar H, Virgen-Montelongo M, Cortez-Alvarez CR, et al. In vitro
interference by acetaminophen, aspirin, and metamizole in serum
measurements of glucose, urea, and creatinine. Clin Biochem. 2015;48:
538–541.
197. Greenberg N, Roberts WL, Bachmann LM, et al. Specificity characteristics
of 7 commercial creatinine measurement procedures by enzymatic and
Jaffe method principles. Clin Chem. 2012;58:391–401.
198. Dilena BA. Bacterial interference with measurement of creatinine in
stored plasma. Clin Chem. 1988;34:1007–1008.
199. Nah H, Lee SG, Lee KS, et al. Evaluation of bilirubin interference and
accuracy of six creatinine assays compared with isotope dilution-liquid
chromatography mass spectrometry. Clin Biochem. 2016;49:274–281.
200. Owen LJ, Keevil BG. Does bilirubin cause interference in Roche creatinine
methods? Clin Chem. 2007;53:370–371.
201. Ali AC, Mihas CC, Campbell JA. Interferences of o-raffinose cross-linked
hemoglobin in three methods for serum creatinine. Clin Chem.
1997;43:1738–1743.
202. Green AJ, Halloran SP, Mould GP, et al. Interference by newer
cephalosporins in current methods for measuring creatinine. Clin
Chem. 1990;36:2139–2140.
203. Swain RR, Briggs SL. Positive interference with the Jaffe reaction by
cephalosporin antibiotics. Clin Chem. 1977;23:1340–1342.
204. Dick JB, Bartlett WA, Ibrahim U, et al. Interference of fluorescein with
creatinine assays. Ann Clin Biochem. 1991;28(Pt 3):311–313.
205. Myers GL, Miller WG, Coresh J, et al. Recommendations for improving
serum creatinine measurement: a report from the Laboratory Working
Group of the National Kidney Disease Education Program. Clin Chem.
2006;52:5–18.
206. Cobbaert CM, Baadenhuijsen H, Weykamp CW. Prime time for enzymatic
creatinine methods in pediatrics. Clin Chem. 2009;55:549–558.
207. Weatherburn MW, Trotman RB, Jackson SH. Specific method for serum
creatinine determination based on ion exchange chromatography and
an automated alkaline picrate reaction—
a proposed reference
method. Clin
Biochem. 1978;11:159–166.
208. Hortin GL, Goolsby K. Lipemia interference with a rate-blanked
creatinine method. Clin Chem. 1997;43:408–410.
209. Carobene A, Ferrero C, Ceriotti F, et al. Creatinine measurement
proficiency testing: assignment of matrix-adjusted ID GC-MS target
values. Clin Chem. 1997;43:1342–1347.
210. Syal K, Srinivasan A, Banerjee D. Streptomycin interference in Jaffe
reaction—possible false positive creatinine estimation in excessive
dose exposure. Clin Biochem. 2013;46:177–179.
211. Schoenmakers CH, Kuller T, Lindemans J, et al. Auto mated enzymatic
methods for creatinine measur ement with special attention to
bilirubin interference. Eur J Clin Chem Clin Biochem. 1993;31:861–868.
212. Sena SF, Syed D, Romeo R, et al. Lidocaine metabolite and creatinine
measurements in the Ektachem 700: steps to minimize its impact on
patient care. Clin Chem. 1988;34:2144 – 2148.
213. Huang JW, Lahey B, Clarkin OJ, et al. A systematic review of the effect of
N-acetylcysteine on serum creatinine and cystatin C measurements.
Kidney Int Rep. 2021;6:396–403.
214. Knezevic CE, Ness MA, Kratz LE, et al. Elevated creatinine in a patient on
IVIG-therapy. Clin Chim Acta. 2018;486:94–97.
215. Natarajan B, Hart T, Smith S, et al. Phenindione interference in enzymatic
creatinine assay—a case report. Clin Nephrol. 2015;83:121–123.
216. Zhang Q, Feng Z, Zhou J, et al. The effect of rheumatoi d factor on three
commercial immunoassays for serum cystatin C. Scand J Clin Lab Invest.
2021;81:112–115.
217. Ismail AA, Walker PL, Cawood ML, et al. Interference in immunoassay is
an underestimated problem. Ann Clin Biochem. 2002;39(Pt 4):366–373.
218. Wauthier L, Plebani M, Favresse J. Interferences in immunoassays: review
and practical algorithm. Clin Chem Lab Med. 2022;60:808–820.
219. Ford L, Berg J. Delay in separating blood samples affects creatinine
measurement using the Roche kinetic Jaffe method. Ann Clin Biochem.
2008;45:83–87.
220.
Miller WG. Estimating glomerular filtration
rate. Clin Chem Lab Med.
2009;47:1017–1019.
221. Grubb A, Blirup-Jensen S, Lindström V, et al. First certified reference
material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem
Lab Med. 2010;48:1619–1621.
222. Fraser CG, Harris EK. Generation and application of data on biological
variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27:409–437.
223. Miller WG, Kaufman HW, Levey AS, et al. National Kidney Foundation
Laboratory Engagement Working Group Recommendations for
Implementing the CKD-EPI 2021 Race-Free Equations for Estimated
Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories.
Clin Chem. 2022;68:511–520.
224. Ardissino G, Testa S, Dacco V, et al. Puberty is associated with increased
deterioration of renal function in patients with CKD: data from the ItalKid
Project. Arch Dis Child. 2012;97:885–888.
225. Gluck CA, Forrest CB, Davies AG, et al. Evaluating kidney function decline
in children with chronic kidney disease using a multi-institutional
electronic health record database. Clin J Am Soc Nephrol. 2023;18:173–182.
226. Drube J, Wan M, Bonthuis M, et al. Clinical practice recommendations for
growth hormone treatment in children with chronic kidney disease. Nat
Rev Nephrol. 2019;15:577–589.
227. Schmitt CP, Shroff RC. Disorders of bone mineral metabolism in chronic
kidney disease. In: Schaefer F, Greenbaum LA, eds. Pediatric Kidney
Disease. Cham: Springer International Publishing; 2023:1631–1668.
228. ESCAPE Trial Group. Strict Blood-Pressure Control and Progression of
Renal Failure in Children. N Engl J Med. 2009;361:1639–1650.
229. Sinha MD, Gu H, Douiri A, et al. Intensive compared with less intensive
blood pressure control to prevent adverse cardiac remodelling in
children with chronic kidney disease (HOT-KID): a parallel-group, open-
label, multicentre, randomised, controlled trial. Lancet Child Adolesc
Health. 2023;7:26 – 36.
230. Atkinson MA, Ng DK, Warady BA, et al. The CKiD study: overview and
summary of findings related to kidney disease progression. Pediatr
Nephrol. 2021;36:527–538.
231. Kang HG, Choi HJ, Han KH, et al. KNOW-Ped CKD (KoreaN cohort study
for outcomes in patients with pediatric CKD): design and methods. BMC
Nephrol. 2016;17:35.
232. Francis A, Didsbury MS, van Zwieten A, et al. Quality of life of children
and adoles cents with chronic kidney disease: a cross-sectional study.
Arch Dis Child. 2019;104:134–140.
233. Stevens
LA, Manzi J, Levey AS, et al. Impact of creatinine calibration on
performance of GFR estimating equations in a pooled individual patient
database. Am J Kidney Dis. 2007;50:21–35.
234. Fabian J, George JA, Etheredge HR, et al. Methods and reporting of
kidney function: a systematic review of studies from sub-Saharan
Africa. Clin Kidney J. 2019;12:778–787.
235. Jessani S, Levey AS, Bux R, et al. Estimation of GFR in South Asians: a
study from the general population in Pakistan. Am J Kidney Dis.
2014;63:49–58.
236. Delanaye P, Schaeffner E, Cozzolino M, et al. The new, race-free, Chronic
Kidney Disease Epidemiology Consortium (CKD-EPI) equation to
estimate glomerular filtration rate: is it applicable in Europe? A
position statement by the European Federation of Clinical Chemistry
and Laboratory Medicine (EFLM). Clin Chem Lab Med. 2022;61:44–47.
237. Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR
estimation: recommendations of the NKF-ASN Task Force on
reassessing the inclusion of race in diagnosing kidney disease. JAm
Soc Nephrol. 2021;32:2994–3015.
238. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate
glomerular filtration rate. Ann Intern Med. 2009;150:604–612.
239. Pierce CB, Munoz A, Ng DK, et al. Age- and sex-dependent clinical
equations to estimate glomerular filtration rates in children and young
adults with chronic kidney disease. Kidney Int. 2021;99:948–956.
240. Pottel H, Björk J, Courbebaisse M, et al. Development and validation of a
modified full age spectrum creatinine-based equation to estimate
glomerular filtration rate: a cross-sectional analysis of pooled data. Ann
Intern Med. 2021;174:183–191.
241. Nyman U, Grubb A, Larsson A, et al. The revised Lund-Malmo GFR
estimating equation outperforms MDRD and CKD-EPI across GFR, age
and BMI intervals in a large Swedish population. Clin Chem Lab Med.
2014;52:815–824.
242. Liu X, Gan X, Chen J, et al. A new modified CKD-EPI equation for Chinese
patients with type 2 diabetes. PLoS One. 2014;9:e109743.
243. Grubb A, Horio M, Hansson LO, et al. Generation of a new cystatin C-
based estimating equation for gl omerular filtration rate by use of 7
assays standardized to the international calibrator. Clin Chem. 2014;60:
974–986.
244.
Zhao L, Li HL, Liu HJ, et al. Validation of the EKFC equation for glomerular
filtration
rate estimation and comparison with the Asian-modified CKD-
EPI equation in Chinese chronic kidney disease patients in an external
study. Renal Fail. 2023;45:2150217.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S299

245. Eneanya ND, Adingwupu OM, Kostelanetz S, et al. Social determinants of
health and their impact on the Black race coefficient in serum creatinine-
based estimation of GFR: secondary analysis of MDRD and CRIC studies.
Clin J Am Soc Nephrol. 2023;18:446–454.
246. Borrell LN, Elhawary JR, Fuentes-Afflick E, et al. Race and genetic ancestry
in medicine—a time for reckoning with racism. N Engl J Med. 2021;384:
474–480.
247. Oni-Orisan A, Mavura Y, Banda Y, et al. Embracing genetic diversity to
improve Black health. N Engl J Med. 2021;384:1163–1167.
248. Agarwal A. Sept 25, 2020 Letter to Chairman Richard Neal (Committee
on Ways and Means) re Race and eGFR. Accessed February 14, 2023.
https://www.asn-online.org/policy/webdocs/20.9.25ASNResponsetoChairman
NealreRaceandeGFR.pdf
249. Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of
using race to estimate kidney function. JAMA. 2019;322:113–114.
250. Gama RM, Kalyesubula R, Fabian J, et al. NICE takes ethnicity out of
estimating kidney function. BMJ. 2021;374:n2159.
251. Griffiths K, Gama RM, Fabian J, et al. Interpreting an estimated
glomerular filtration rate (eGFR) in people of black ethnicities in the
UK. BMJ. 2023;380:e073353.
252. Parekh RS, Perl J, Auguste B, et al. Elimination of race in estimates of
kidney function to provide unbiased clinical management in Canada.
CMAJ. 2022;194:E421–E423.
253. Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight—reconsidering the
use of race correction in clinical algorithms. NEnglJMed. 2020;383:874–882.
254. Warren E, Booker C, Wyden R, et al. A review of the use of race-based
clinical algorithms in standard medical practices. Agency for
Healthcare Research and Quality; 2020. Accessed May 8, 2023. https://
www.warren.senate.gov/imo/media/doc/9.22.2020%20Letter%20to%20
AHRQ%20re%20Use%20of%20Race%20in%20Clinical%20Algorithms.pdf
255. Delgado C, Baweja M, Burrows NR, et al. Reassessing the inclusion of race
in diagnosing kidney diseases: an interim report from the NKF-ASN Task
Force. J Am Soc Nephrol. 2021;32:1305–1317.
256. Gansevoort RT, Anders HJ, Cozzolino M, et al. What should European
nephrology do with the new CKD-EPI equation? Nephrol Dial
Transplant. 2023;38:1–6.
257. Nyman U, Bjork J, Berg U, et al. The modified CKiD study estimated GFR
equations for children and young adults under 25 years of age:
performance in a european multicenter cohort. Am J Kidney Dis.
2022;80:807–810.
258.
Heathcote KL, Wilson MP, Quest DW, et al. Prevalence and duration of
exerci
se induced albuminuria in healthy people. Clin Invest Med.
2009;32:E261–E265.
259. Carter JL, Tomson CR, Stevens PE, et al. Does urinary tract infection cause
proteinuria or microalbuminuria? A systematic review. Nephrol Dial
Transplant. 2006;21:3031–3037.
260. McTaggart MP, Stevens PE, Price CP, et al. Investigation of apparent non-
albuminuric proteinuria in a primary care population. Clin Chem Lab Med .
2013;51:1961–1969.
261. Howey JE, Browning MC, Fraser CG. Selecting the optimum specimen for
assessing slight albuminuria, and a strategy for clinical investigation: novel
uses of data on biological variation. Clin Chem. 1987;33:2034–2038.
262. Ballantyne FC, Gibbons J, O’Reilly DS. Urine albumin should replace total
protein for the assessment of glomerular proteinuria. Ann Clin Biochem.
1993;30(Pt 1):101–103.
263. Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected
and measured? Ann Clin Biochem. 2009;46:205–217.
264. Newman DJ, Thakkar H, Medcalf EA, et al. Use of urine albumin measurement
as a replacement for total protein. Clin Nephrol. 1995;43:104–109.
265. Dawnay A, Wilson AG, Lamb E, et al. Microalbumi nuria in systemic
sclerosis. Ann Rheum Dis. 1992;51:384–388.
266. Gross JL, de Azevedo MJ, Silveiro SP, et al. Diabetic nephropathy:
diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–176.
267. Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney
function independently predict cardiovascular and renal outcomes in
diabetes. J Am Soc Nephrol. 2009;20:1813–1821.
268. Shihabi ZK, Konen JC, O’Connor ML. Albuminuria vs urinary total
protein for detecting chronic renal disorders. Clin Chem. 1991;37:621–624.
269. Martin H. Laboratory measurement of urine albumin and urine total
protein in screening for proteinuria in chronic kidney disease. Clin
Biochem Rev. 2011;32:97–102.
270. Waugh J, Bell SC, Kilby M, et al. Effect of concentration and biochemical
assay on the accuracy of urine dipsticks in hypertensive pregnancies.
Hypertens Pregnancy. 2001;20:205–217.
271. Waugh J, Bell SC, Kilby MD, et al. Urine protein estimation in
hypertensive pregnancy: which thresholds and laboratory assay best
predict
clinical outcome? Hypertens Pregnancy. 2005;24:291–302.
272. El Nahas M. Cardio-kidney-damage: a unifying concept. Kidney Int.
2010;78:14–18.
273. McElderry LA, Tarbit IF, Cassells-Smith AJ. Six methods for urinary protein
compared. Clin Chem. 1982;28:356–360.
274. Nishi HH, Elin RJ. Three turbidimetric methods for determining total
protein compared. Clin Chem. 1985;31:1377–1380.
275. Sedmak JJ, Grossberg SE. A rapid, sensitive, and versatile assay for
protein using Coomassie brilliant blue G250. Anal Biochem. 1977;79:
544–552.
276. de Keijzer MH, Klasen IS, Branten AJ, et al. Infusion of plasma expanders
may lead to unexpected results in urinary protein assays. Scand J Clin
Lab Invest. 1999;59:133–137.
277. Marshall T, Williams KM. Extent of aminoglycoside interference in the
pyrogallol red-molybdate protein assay depends on the concentration
of sodium oxalate in the dye reagent. Clin Chem. 2004;50:934–935.
278. Chambers RE, Bullock DG, Whicher JT. External quality assessment of
total urinary protein estimation in the United Kingdom. Ann Clin
Biochem. 1991;28(Pt 5):467–473.
279. Heick HM, Begin-Heick N, Acharya C, et al. Automated determination of
urine and cerebrospinal fluid proteins with Coomassie brilliant blue and
the Abbott ABA-100. Clin Biochem . 1980;13:81–83.
280. Marshall T, Williams KM. Total protein determination in urine: elimination
of a differential response between the Coomassie blue and pyrogallol
red protein dye-binding assays. Clin Chem. 2000;46:392–398.
281. Ginsberg JM, Chang BS, Matarese RA, et al. Use of single voided urine
samples to estimate quantitative proteinuria. N Engl J Med. 1983;309:
1543–1546.
282. Price CP, Newall RG, Boyd JC. Use of protein:creatinine ratio
measurements on random urine samples for prediction of signifi cant
proteinuria: a systematic review. Clin Chem. 2005;51:1577–1586.
283. Beetham R, Cattell WR. Proteinuria: pathophysiology, significance and
recommendations for measurement in clinical practice. Ann Clin
Biochem
. 1993;30(Pt 5):425–434.
284. Keane
WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment,
detection, elimination (PARADE): a position paper of the National
Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010.
285. Claudi T, Cooper JG. Comparison of urinary albumin excretion rate in
overnight urine and albumin creatinine ratio in spot urine in diabetic
patients in general practice. Scand J Prim Health Care. 2001;19:247–248.
286. Gatling W, Knight C, Mullee MA, et al. Microalbuminuria in diabetes: a
population study of the prevalence and an assessment of three
screening tests. Diabet Med. 1988;5:343–347.
287. Hutchison AS, O’Reilly DS, MacCuish AC. Albumin excretion rate, albumin
concentration, and albumin/creatinine ratio compared for screening
diabetics for slight albuminuria. Clin Chem. 1988;34:2019–2021.
288. Marshall SM. Screening for microalbuminuria: which measurement?
Diabet Med. 1991;8:706–711.
289. Marshall SM, Alberti KG. Screening for early diabetic nephropathy. Ann
Clin Biochem. 1986;23(Pt 2):195–197.
290. Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and
reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38.
291. Chitalia VC, Kothari J, Wells EJ, et al. Cost-benefit analysis and prediction
of 24-hour proteinuria from the spot urine protein-creatinine ratio. Clin
Nephrol. 2001;55:436–447.
292. Cote AM, Brown MA, Lam E, et al. Diagnostic accuracy of urinary spot
protein:creatinine ratio for proteinuria in hypertensive pregnant
women: systematic review. BMJ. 2008;336:1003–1006.
293. Dyson EH, Will EJ, Davison AM, et al. Use of the urinary protein creatinine
index to assess proteinuria in renal transplant patients. Nephrol Dial
Transplant. 1992;7:450–452.
294. Leanos-Miranda A, Marquez-Acosta J, Romero-Arauz F, et al. Protein:
creatinine ratio in random urine samples is a reliable marker of
increased 24-hour protein excretion in hospitalized women with
hypertensive disorders of pregnancy. Clin Chem. 2007;53:1623–1628.
295. Lemann J Jr, Doumas BT. Proteinuria in health and disease assessed by
measuring the urinary protein/creatinine ratio. Clin Chem. 1987;33:297–
299.
296. Ralston SH, Caine N, Richards I, et al. Screening for proteinuria in a
rheumatology clinic: comparison of dipstick testing, 24 hour urine
quantitative protein, and protein/creatinine ratio in random urine
samples. Ann Rheum Dis. 1988;47:759–763.
references www.kidney-international.org
S300
Kidney International (2024) 105 (Suppl 4S), S117–S314

297. Ruggenenti P, Gaspari F, Perna A, et al. Cross sectional longitudinal study
of spot morning urine protein:creatinine ratio, 24 hour urine protein
excretion rate, glomerular filtration rate, and end stage renal failure in
chronic renal disease in patients without diabetes. BMJ. 1998;316:504–
509.
298. Saudan PJ, Brown MA, Farrell T, et al. Improved methods of assessing
proteinuria in hypertensive pregnancy. Br J Obstet Gynaecol. 1997;104:
1159–1164.
299. Abdelmalek JA, Gansevoort RT, Lambers Heerspink HJ, et al. Estimated
albumin excretion rate versus urine albumin-creatinine ratio for the
assessment of albuminuria: a diagnostic test study from the
Prevention of Renal and Vascular Endstage Disease (PREVEND) Study.
Am J Kidney Dis. 2014;63:415–421.
300. Fotheringham J, Campbell MJ, Fogarty DG, et al. Estimated albumin
excretion rate versus urine albumin-creatinine ratio for the estimation
of measured albumin excretion rate: derivation and validation of an
estimated albumin excretion rate equation. Am J Kidney Dis. 2014;63:
405–414.
301. Newman DJ, Pugia MJ, Lott JA, et al. Urinary protein and albumin
excretion corrected by creatinine and specific gravity. Clin Chim Acta.
2000;294:139–155.
302. Rehman Z, Franks WT, Nguyen B, et al. Discovering the solid-state
secrets of lorlatinib by NMR crystallography: to hydrogen bond or not
to hydrogen bond. J Pharm Sci. 2023;112:1915–1928.
303. Becherucci F, Roperto RM, Materassi M, et al. Chronic kidney disease in
children. Clin Kidney J. 2016;9:583–591.
304. American Diabetes Association Professional Practice Committee. 14.
Children and adolescents: standards of medical care in diabetes-2022.
Diabetes Care. 2022;45:S208–S231.
305. Houser MT, Jahn MF, Kobayashi A, et al. Assessment of urinary protein
excretion in the adolescent: effect of body position and exercise. J
Pediatr. 1986;109:556–561.
306. Chavers BM, Rheault MN, Foley RN. Kidney function reference values in
US adolescents: National Health and Nutrition Examination Survey 1999-
2008. Clin J Am Soc Nephrol. 2011;6:1956–1962.
307. Larkins NG, Kim S, Carlin JB, et al. Albuminuria: population epidemiology
and concordance in Australian children aged 11–12 years and their
parents. BMJ Open. 2019;9:75–84.
308. Rademacher ER, Sinaiko AR. Albuminuria in children. Curr Opin Nephrol
Hypertens. 2009;18:246–251.
309. Tsioufis C, Mazaraki A, Dimitriadis K, et al. Microalbuminuria in the
paediatric age: current knowledge and emerging questions. Acta
Paediatr.
2011;100:1180–1184.
310. Emma F, Goldstein S, Bagga A, et al. Pediatric Nephrology. 8th ed.
Springer; 2022.
311. Brinkman JW, de Zeeuw D, Duker JJ, et al. Falsely low urinary albumin
concentrations after prolonged frozen storage of urine samples. Clin
Chem. 2005;51:2181–2183.
312. Sacks DB, Arnold M, Bakris GL, et al. Executive summary: guidelines and
recommendations for laboratory analysis in the diagnosis and
management of diabetes mellitus. Clin Chem. 2011;57:793–798.
313. Seegmiller JC, Miller WG, Bachmann LM. Moving toward standardization
of urine albumin measurements. EJIFCC. 2017;28:258–267.
314. National Institute of Standards and Technology. Certification of Standard
Reference Material 2925 Recombinant Human Serum Albumin Solution
(Primary Reference Calibrator for Urine Albumin) (Frozen). U.S.
Department of Commerce, NIST; 2020.
315. Carter JL, Parker CT, Stevens PE, et al. Biological variation of plasma and
urinary markers of acute kidney injury in patients with chronic kidney
disease. Clin Chem. 2016;62:876–883.
316. National Institute for Health and Care Excellence. Point-of-care creatinine
devices to assess kidney function before CT imaging with intravenous
contrast. NICE Guideline [NG37]. NICE; 2019.
317. Batte A, Murphy KJ, Namazzi R, et al. Evaluating kidney function using a
point-of-care creatinine test in Ugandan children with severe malaria: a
prospective cohort study. BMC Nephrol. 2021;22:369.
318. McTaggart MP, Newall RG, Hirst JA, et al. Diagnostic accuracy of point-of-
care tests for detecting albuminuria: a systematic review and meta-
analysis. Ann Intern Med. 2014;160:550–557.
319. Abitbol C, Zilleruelo G, Freundlich M, et al. Quantitation of proteinuria
with urinary protein/creatinine ratios and random testing with
dipsticks in nephrotic children. J Pediatr. 1990;116:243–247.
320. Agardh CD. A new semiquantitative rapid test for screening for
microalbuminuria. Practical Diabetes. 1993;10:146–147.
321. Agarwal R, Panesar A, Lewis RR. Dipstick proteinuria: can it guide
hypertension management? Am J Kidney Dis. 2002;39:1190–1195.
322. Arora S, Long T, Menchine M. Test characteristics of urine dipstick for
identifying renal insufficiency in patients with diabetes. West J Emerg
Med. 2011;12:250–253.
323. Chang
CC, Su MJ, Ho JL, et al. The efficacy of semi-quantitative urine
protein-to-creatinine (P/C) ratio for the detection of significant
proteinuria in urine specimens in health screening settings.
Springerplus. 2016;5:1791.
324. Cho MC, Ji M, Kim SY, et al. Evaluation of the URiSCAN super cassette
ACR semiquantitative urine dipstick for microalbuminuria screening. J
Clin Lab Anal. 2014;28:281–286.
325. Collier G, Greenan MC, Brady JJ, et al. A study of the relationship
between albuminuria, proteinuria and urinary reagent strips. Ann Clin
Biochem. 2009;46:247–249.
326. Collins AC, Vincent J, Newall RG, et al. An aid to the early detection and
management of diabetic nephropathy: assessment of a new point of
care microalbuminuria system in the diabetic clinic. Diabet Med.
2001;18:928–932.
327. Cortés-Sanabria L, Martínez-Ramírez HR, Hernández JL, et al. Utility of the
Dipstick Micraltest II in the screening of microalbuminuria of diabetes
mellitus type 2 and essential hypertension. Rev Invest Clin. 2006;58:
190–197.
328. Croal BL, Mutch WJ, Clark BM, et al. The clinical application of a urine
albumin:creatinine ratio point-of-care device. Clin Chim Acta. 2001;307:
15–21.
329. Currin SD, Gondwe MS, Mayindi NB, et al. Diagnostic accuracy of
semiquantitative point of care urine albumin to creatinine ratio and
urine dipstick analysis in a primary care resource limited setting in
South Africa. BMC Nephrol. 2021;22:103.
330. Dajak M, Bonti
c A, Ugnjatovi
c S, et al. [Evaluation of methods for rapid
microalbuminuria screening in kidney diseased patients]. Srp Arh Celok
Lek. 2012;140:173–178 [in Serbian].
331. Davidson MB, Bazargan M, Bakris G, et al. ImmunoDip: an improved
screening method for microalbuminuria. Am J Nephrol. 2004;24:284–288.
332. Davidson MB, Smiley JF. Relationship between dipstick positive
proteinuria and albumin:creatinine ratios. J Diabetes Complications.
1999;13:52–55.
333. de Grauw WJ, van de Lisdonk EH, van de Hoogen HJ, et al. Screening for
microalbuminuria in type 2 diabetic patients: the evaluation of a dipstick
test in general practice. Diabet Med. 1995;12:657–663.
334. Fernández Fernández I, Páez Pinto JM, Hermosín Bono T, et al. Rapid
screening test evaluation for microalbu minuria in diabetes mellitus.
Acta Diabetol. 1999;35:199–202.
335. Gai
M, Motta D, Giunti S, et al. Comparison between 24-h proteinuria,
urinary protein/creatinine ratio and dipstick test in patients with
nephropathy: patterns of proteinuria in dipstick-negative patients.
Scand J Clin Lab Invest. 2006;66:299–307.
336. Garcia C, Bordier L, Burnat P, et al. [Urinary dipsticks must not be used to
detect diabetes-induced incipient nephropathy]. Presse Med. 2006;35:
1117–1121 [in French].
337. Gilbert RE, Akdeniz A, Jerums G. Semi-quantitative determination of
microalbuminuria by urinary dipstick. Aust N Z J Med. 1992;22:334–337.
338. Gilbert RE, Akdeniz A, Jerums G. Detection of microalbuminuria in
diabetic patients by urin ary dipstick. Diabetes Res Clin Pract. 1997;35:
57–60.
339. Graziani MS, Gambaro G, Mantovani L, et al. Diagnostic accuracy of a
reagent strip for assessing urinary albumin excretion in the general
population. Nephrol Dial Transplant. 2008;24:1490–1494.
340. Guy M, Newall R, Borzomato J, et al. Use of a first-line urine protein-to-
creatinine ratio strip test on random urines to rule out proteinuria in
patients with chronic kidney disease. Nephrol Dial Transplant. 2008;24:
1189–1193.
341. Guy M, Newall R, Borzomato J, et al. Diagnostic accuracy of the urinary
albumin: creatinine ratio determined by the CLINITEK Microalbumin and
DCA 2000þ for the rule-out of albu minuria in chronic kidney disease.
Clin Chim Acta. 2008;399:54–58.
342. Hasslacher C, Muller P, Schlipfenbacher RL. Results of a multicentre study
for the determination of microalbumi nuria with Micral-Test. Klinisches
Labor. 1995;41:441 – 447.
343. Hodel NC, Hamad A, Reither K, et al. Comparison of two different
semiquantitative urinary dipstick tests with albumin-to-creatinine ratio
for screening and classification of albuminuria according to KDIGO. A
diagnostic test study. Diagnostics (Basel). 2021;11:81.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S301

344. Incerti J, Zelmanovitz T, Camargo JL, et al. Evaluation of tests for
microalbuminuria screening in patients with diabetes. Nephrol Dial
Transplant. 2005;20:2402–2407.
345. Kaiser C, Bergel F, Doehring-Schwerdtfeger E, et al. Urine test strips:
reliability of semi-quantitative findings under tropical conditions.
Pediatr Nephrol. 1992;6:145–148.
346. Khawali C, Andriolo A, Ferreira SR. Comparison of methods for urinary
albumin determination in patients with type 1 diabetes. Braz J Med
Biol Res. 2002;35:337–343.
347. Kim Y, Park S, Kim MH, et al. Can a semi-quantitative method replace the
current quantitative method for the annual screening of microalbuminuria
in patients with diabetes? Diagnostic accuracy and cost-saving analysis
considering the potential health burden. PLoS One. 2020;15:e0227694.
348. Le Floch JP, Marre M, Rodier M, et al. Interest of Clinitek Microalbumin in
screening for microalbuminuria: results of a multicentre study in 302
diabetic patients. Diabetes Metab. 2001;27:36–39.
349. Leong SO, Lui KF, Ng WY, et al. The use of semi-quantitative urine test-
strip (Micral Test) for microalbu minuria screening in patients with
diabetes mellitus. Singapore Med J. 1998;39:101–103.
350. Lim D, Lee DY, Cho SH, et al. Diagnostic accuracy of urine dipstick for
proteinuria in older outpatients. Kidney Res Clin Pract. 2014;33:199–203.
351. Lim S, Yu HJ, Lee S, et al. Evaluation of the URiSCAN 2 ACR Strip to
estimate the urine albumin/creatinine ratios. J Clin Lab Anal. 2017;32:
e22289.
352. Lin CJ, Chen HH, Pan CF, et al. The characteristics of new semi-
quantitative method for diagnosing proteinuria by using random urine
samples. J Clin Lab Anal. 2011;25:14–19.
353. Lloyd MM, Kuyl J, van Jaarsveld H. Evaluation of point-of-care tests for
detecting microalbuminuria in diabetic patients. S Afr Fam Pract.
2011;53:281–286.
354. Marshall SM, Shearing PA, Alberti KG. Micral-test strips evaluated for
screening for albuminuria. Clin Chem. 1992;38:588–591.
355. Masimango MI, Hermans MP, Malembaka EB, et al. Impact of rural versus
urban setting on kidney markers: a cross-sectional study in South-Kivu,
DRCongo. BMC Nephrol. 2021;22:234.
356. Masimango MI, Sumaili EK, Jadoul M, et al. Prevalence of
microalbuminuria and diagnostic value of dipstick proteinuria in
outpatients from HIV clinics in Bukavu, the Democratic Republic of
Congo. BMC Nephrol. 2014;15:146.
357. McTaggart MP, Price CP, Pinnock RG, et al. The diagnostic accuracy of a
urine albumin-creatinine ratio point-of-care test for detection of
albuminuria in primary care. Am J Kidney Dis. 2012;60:787–794.
358.
Meinhardt U, Ammann RA, Flück C, et al. Microalbuminuria in diabetes
me
llitus: efficacy of a new screening method in comparison with timed
overnight urine collection. JDiabetesComplications. 2003;17:254–257.
359. Minetti EE, Cozzi MG, Granata S, et al. Accuracy of the urinary albumin
titrator stick ’Micral-Test’ in kidney-disease patients. Nephrol Dial
Transplant. 1997;12:78–80.
360. Naruse M, Mukoyama M, Morinaga J, et al. Usefulness of the quantitative
measurement of urine protein at a community-based health checkup: a
cross-sectional study. Clin Exp Nephrol. 2019;24:45–52.
361. Olivarius ND, Mogensen CE. Danish general practitioners’ estimation of
urinary albumin concentration in the detection of proteinuria and
microalbuminuria. Br J Gen Pract. 1995;45:71–73.
362. Osta V, Natoli V, Diéguez S. [Evaluation of two rapid tests for the
determination of microalbuminuria and the urinary albumin/creatinine
ratio]. An Pediatr (Barc). 2003;59:131–137 [in Spanish].
363. Oyaert M, Delanghe JR. Semiquantitative, fully automated urine test strip
analysis. J Clin Lab Anal. 2019;33:e22870.
364. Parker JL, Kirmiz S, Noyes SL, et al. Reliability of urinalysis for
identification of proteinuria is reduced in the presence of other
abnormalities including high specific gravity and hematuria. Urol
Oncol. 2020;38:853.e859–853.e915.
365. Parsons M, Newman DJ, Pugia M, et al. Performance of a reagent strip
device for quantitation of the urine albumin: creatinine ratio in a point
of care setting. Clin Nephrol. 1999;51:220–227.
366. Parsons MP, Newman DJ, Newall RG, et al. Validation of a point-of-care
assay for the urinary albumin:creatinine ratio. Clin Chem. 1999;45:414–
417.
367. Penders J, Fiers T, Delanghe JR. Quantitative evaluation of urinalysis test
strips. Clin Chem. 2002;48:2236–2241.
368. Poulsen PL, Mogensen CE. Clinical evaluation of a test for immediate and
quantitative determ ination of urinary albumin-to-creatinine ratio. A brief
report. Diabetes Care. 1998;21:97–98.
369. Pugia MJ, Lott JA, Kajima J, et al. Screening school children for
albuminuria, proteinuria and occult blood with dipsticks. Clin Chem
Lab Med. 1999;37:149–157.
370. Sakai N, Fuchigami H, Ishizuka T, et al. Relationship between a
urine protein-to-creatinine ratio of 150 mg/gram creatinine and
dipstick grade in the health checkup: substantial number of false-
negative
results for chronic kidney disease. Tokai J Exp Clin Med.
2019;44:118–123.
371. Salinas M, López-Garrigós M, Flores E, et al. Urinary albumin strip assay
as a screening test to replace quantitative technology in certain
conditions. Clin Chem Lab Med. 2018;57:204–209.
372. Sarafidis PA, Riehle J, Bogojevic Z, et al. A comparative evaluation of
various methods for microalbuminuria screening. Am J Nephrol.
2007;28:324–329.
373. Shephard MD, Barratt LJ, Simpson-Lyttle W. Is the Bayer DCA 2000
acceptable as a screening instrument for the early detection of renal
disease? Ann Clin Biochem. 1999;36(Pt 3):393–394.
374. Siedner MJ, Gelber AC, Rovin BH, et al. Diagnostic accuracy study of
urine dipstick in relation to 24-hou r measurement as a screening tool
for proteinuria in lupus nephritis. J Rheumatol. 2007;35:84–90.
375. Spooren PF, Lekkerkerker JF, Vermes I. Micral-test: a qualitative dipstick
test for micro-albuminuria. Diabetes Res Clin Pract. 1992;18:83–87.
376. Szymanowicz A, Blanc-Bernard E, Roche C, et al. Evaluation of Micral
Test for the screening of the microalbuminuria in point of care
testing. Immuno-Analyse Biologie Specialisee. 2008;23:109–115.
377. Tiu SC, Lee SS, Cheng MW. Comparison of six commercial techniques in
the measurement of microalbuminuria in diabetic patients. Diabetes
Care. 1993;16:616 – 620.
378. Tsujikawa H, Machii R, Hiratsuka N, et al. [Evaluation of novel test strip to
measure albumin and creatinine in urine]. Rinsho Byori . 2005;53:111–117
[in Japanese].
379. Usui T, Yoshida Y, Nishi H, et al. Diagnostic accuracy of urine dipstick for
proteinuria category in Japanese workers. Clin Exp Nephrol. 2019;24:151–
156.
380. Yanagisawa N, Muramatsu T, Koibuchi T, et al. Prevalence of chronic
kidney disease and poor diagnostic accuracy of dipstick proteinuria in
human immunodeficiency virus-infected individuals: a multicenter
study in Japan. Open Forum Infect Dis. 2018;5:ofy216.
381. Yang CJ, Chen DP, Wen YH, et al. Evaluation the diagnostic accuracy of
albuminuria detection in semi-quantitative urinalysis. Clin Chim Acta.
2020;510:177–180.
382. Kouri T, Nokelainen P, Pelkonen V, et al. Evaluation of the ARKRAY
AUTION Eleven reflectometer in detecting microalbuminuria with
AUTION Screen test strips and proteinuria with AUTION Sticks 10PA
strips. Scand
J Clin Lab Invest. 2008;69:52 – 64.
383. Nagrebetsky A, Jin J, Stevens R, et al. Diagnostic accuracy of urine
dipstick testing in screening for microalbuminuria in type 2 diabetes: a
cohort study in primary care. Fam Pract. 2012;30:142–152.
384. Nah EH, Cho S, Kim S, et al. Comparison of urine albumin-to-creatinine
ratio (ACR) between ACR strip test and quantitative test in prediabetes
and diabetes. Ann Lab Med. 2016;37:28–33.
385. Shin JI, Chang AR, Grams ME, et al. Albuminuria testing in hypertension
and diabetes: an individual-participant data meta-analysis in a Global
Consortium. Hypertension. 2021;78:1042–1052.
386. Pantalone KM, Ji X, Kong SX, et al. Unmet needs and opportunities for
optimal management of patients with type 2 diabetes and chronic
kidney disease. J Diabetes Complications. 2023;37:108418.
387. Kim HS, Ng DK, Matheson MB, et al. Association of puberty with changes
in GFR in children with CKD. Am J Kidney Dis. 2022;79:131–134.
388. Gianluigi A, Sara T, Valeria D, et al. Puber ty is associated with increased
deterioration of renal function in patients with CKD: data from the ItalKid
Project. Arch Dis Child. 2012;97:885–888.
389. Oshima M, Jardine MJ, Agarwal R, et al. Insights from CREDENCE trial
indicate an acute drop in estimated glomerular filtration rate during
treatment with canagliflozin with implications for clinical practice.
Kidney Int. 2021;99:999–1009.
390. Kraus BJ, Weir MR, Bakris GL, et al. Characterization and implications of
the initial estimated glomerular filtration rate ’dip’ upon sodium-glucose
cotransporter-2 inhibition with empagliflozin in the EMPA-REG
OUTCOME trial. Kidney Int. 2021;99:750–762.
391. Norris KC, Smoyer KE, Rolland C, et al. Albuminuria, serum creatinine,
and estimated glomerular filtration rate as predictors of cardio-renal
outcomes in patients with type 2 diabetes mellitus and kidney
disease: a systematic literature review. BMC Nephrol. 2018;19:36.
references www.kidney-international.org
S302
Kidney International (2024) 105 (Suppl 4S), S117–S314

392. Lambers Heerspink HJ, Gansevoort RT. Albuminuria is an appropriate
therapeutic target in patients with CKD: the pro view. Clin J Am Soc
Nephrol. 2015;10:1079–1088.
393. Fuhrman DY, Schneider MF, Dell KM, et al. Albuminuria, proteinuria, and
renal disease progression in children with CKD. Clin J Am Soc Nephrol.
2017;12:912–920.
394. Harambat J, Kunzmann K, Azukaitis K, et al. Metabolic acidosis is
common and associates with disease progression in children with
chronic kidney disease. Kidney Int. 2017;92:1507–1514.
395. van den Belt SM, Heerspink HJL, Kirchner M, et al. Disc ontinuation of
RAAS inhibition in children with advanced CKD. Clin J Am Soc Nephrol.
2020;15:625–632.
396. Ardissino G, Testa S, Daccò V, et al. Proteinuria as a predictor of disease
progression in children with hypodysplastic nephropathy. Data from the
Ital Kid Project. Pediatr Nephrol . 2004;19:172–177.
397. Kamath N, Iyengar A, George N, et al. Risk factors and rate of progression
of CKD in children. Kidney Int Rep. 2019;4:1472 – 1477.
398. Ishikura K, Uemura O, Hamasaki Y, et al. Progression to end-stage kidney
disease in Japanese children with chronic kidney disease: results of a
nationwide prospective cohort study. Nephrol Dial Transplant. 2014;29:
878–884.
399. Rigatto C, Sood MM, Tangri N. Risk prediction in chronic kidney disease:
pitfalls and caveats. Curr Opin Nephrol Hypertens. 2012;21:612–618.
400. Stevens PE, Levin A, Kidney Disease. Improving Global Outcomes
Chronic Kidney Disease Guideline Development Work Group Members.
Evaluation and management of chronic kidney disease: synopsis of
the kidney disease: improving global outcomes 2012 clinical practice
guideline. Ann Intern Med. 2013;158:825–830.
401. Chen TK, Hoenig MP, Nitsch D, et al. Advances in the management of
chronic kidney disease. BMJ. 2023;383:074216.
402. Grams M, Sang Y, Ballew S, et al. TH-PO890. Risk prediction: CKD staging
is the beginning, not the end. J Am Soc Nephrol . 2022;33:301.
403. The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al.
Empagliflozin in patients with chronic kidney disease. N Engl J Med.
2023;388:117–127.
404. Rifkin DE. Chronicle of a Death Foretold: can studying death help us care
for the living? Clin J Am Soc Nephrol. 2020;15:883–885.
405. Ramspek CL, de Jong Y, Dekker FW, et al. Towards the best kidney failure
prediction tool: a systematic review and selection aid. Nephrol
Dial
Transplant. 2020;35:1527–1538.
406. Tangri N, Kitsios GD, Inker LA, et al. Risk prediction models for patients
with chronic kidney disease: a systematic review. Ann Intern Med.
2013;158:596–603.
407. Grams ME, Brunskill NJ, Ballew SH, et al. The kidney failure risk equation:
evaluation of novel input variables including eGFR estimated using the
CKD-EPI 2021 equation in 59 cohorts. J Am Soc Nephrol. 2023;34:482–
494.
408. Major RW, Shepherd D, Medcalf JF, et al. The kidney failure risk equation
for prediction of end stage renal disease in UK primary care: an external
validation and clinical impact projection cohort study. PLoS Med.
2019;16:e1002955.
409. Zacharias HU, Altenbuchinger M, Schultheiss UT, et al. A predictive
model for progression of CKD to kidney failure based on routine
laboratory tests. Am J Kidney Dis. 2022;79:217–230.e1.
410. Schroeder EB, Yang X, Thorp ML, et al. Predicting 5-year risk of RRT in
stage 3 or 4 CKD: development and external validation. Clin J Am Soc
Nephrol. 2017;12:87–94.
411. Landray MJ, Thambyrajah J, McGlynn FJ, et al. Epidemiological
evaluation of known and suspected cardiovascular risk factors in
chronic renal impairment. Am J Kidney Dis. 2001;38:537–546.
412. Che M, Iliescu E, Thanabalasingam S, et al. Death and dialysis following
discharge from chronic kidney disease clinic: a retrospective cohort
study. Can J Kidney Health Dis. 2022;9:20543581221118434.
413. Hemmelgarn BR, Smekal MD, Weaver RG, et al. Implementation and
evaluation of a risk-based approach to guide chronic kidney disease
care: protocol for a multiphase mixed-methods study. Can J Kidney
Health Dis. 2018;5:2054358117753618.
414. Drawz PE, Goswami P, Azem R, et al. A simple tool to predict end-stage
renal disease within 1 year in elderly adults with advanced chronic
kidney disease. J Am Geriatr Soc. 2013;61:762–768.
415. National Institute for Health and Care Excellence. Evidence review for the
best combination of measures to identify increased risk of progression in
adults, children and young people: chronic kidney disease. NICE Evidence
Reviews Collection. NICE; 2021.
416. Winnicki E, McCulloch CE, Mitsnefes MM, et al. Use of the kidney failure
risk equation to determine the risk of progression to end-stage renal
disease in children with chronic kidney disease. JAMA Pediatr.
2018;172:174–180.
417. Warady BA, Abraham AG, Schwartz GJ, et al. Predictors of rapid
progression of glomerular and nonglomerular kidney disease in
children and adolescents: the Chronic Kidney Disease in Children
(CKiD) cohort. Am J Kidney Dis. 2015;65:878–888.
418. Furth SL, Pierce C, Hui WF, et al. Estimating time to ESRD in children with
CKD. Am J Kidney Dis. 2018;71:783–792.
419. Menon G, Pierce CB, Ng DK, et al. Revisiting the application of an adult
kidney failure risk prediction equation to children with CKD. Am
J Kidney
Dis. 2023;81:734–737.
420. National Institute for Health and Care Excellence. Chronic kidney
disease: assessment and management. NICE Guideline [NG203]. Report
no. 978-1-4731-4233-6. NICE. 2021.
421. Hingwala J, Wojciechowski P, Hiebert B, et al. Risk-based triage for
nephrology referrals using the kidney failure risk equation. Can J
Kidney Health Dis. 2017;4:2054358117722782.
422. Smekal MD, Tam-Tham H, Finlay J, et al. Patient and provider experience
and perspectives of a risk-based approach to multidisciplinary chronic
kidney disease care: a mixed methods study. BMC Nephrol. 2019;20:110.
423. Lok CE, Huber TS, Lee T, et al. KDOQI clinical practice guideline for
vascular access: 2019 update. Am J Kidney Dis. 2020;75(suppl 2):S1–S164.
424. Grams ME, Brunskill NJ, Ballew SH, et al. Development and validation of
prediction models of adverse kidney outcomes in the population with
and without diabetes. Diabetes Care. 2022;45:2055–2063.
425. Chan L, Nadkarni GN, Fleming F, et al. Derivation and validation of a
machine learning risk score using biomarker and electronic patient
data to predict progression of diabetic kidney disease. Diabetologia.
2021;64:1504–1515.
426. Cornec-Le Gall E, Audrezet MP, Rousseau A, et al. The PROPKD score: a
new algorithm to predict renal survival in autosomal dominant
polycystic kidney disease. J Am Soc Nephrol. 2016;27:942–951.
427. Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of
autosomal dominant polycystic kidney disease: a simple model
for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26:160–
172.
428. Barbour SJ, Coppo R, Zhang H, et al. Evaluating a new international risk-
prediction tool in IgA nephropathy. JAMA Intern Med. 2019;179:942–952.
429. Berthoux F, Mohey H, Laurent B, et al. Predicting the risk for dialysis or
death in IgA nephropathy. J Am Soc Nephrol. 2011;22:752–761.
430. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of
QRISK3 risk prediction algorithms to estimate future risk of
cardiovascular disease: prospective cohort study. BMJ. 2017;35 7:j2099.
431. Matsushita K, Jassal SK, Sang Y, et al. Incorporating kidney disease measures
into cardiovascular risk prediction: development and validation in 9 million
adults from 72 datasets. EClinicalMedicine. 2020;27:100552.
432. Bansal N, Katz R, De Boer IH, et al. Development and validation of a
model to predict 5-year risk of death without ESRD among older
adults with CKD. Clin J Am Soc Nephrol. 2015;10:363–371.
433. Khan SS, Coresh J, Pencina MJ, et al. Novel prediction equations for
absolute risk assessment of total cardiovascular disease incorporating
cardiovascular-kidney-metabolic health: a scientific statement from the
American Heart Association.
Circulation.
2023;148:1982–2004.
434. Khan SS, Matsushita K, Sang S, et al. Development and validation of the
American Heart Association’s PREVENT equations. Circulation. 2024;149:
430–449.
435. Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet.
2017;389:1238–1252.
436. Kidney Disease: Improving Global Outcomes Anemia Work Group.
KDIGO clinical practice guideline for anemia in chronic kidney disease.
Kidney Int Suppl. 2012;2:S1–S335.
437. Doll R, Peto R, Boreham J, et al. Mortality in relation to smoking: 50 years’
observations on male British doctors. BMJ. 2004;328:1519.
438. Jennings C, Kotseva K, De Bacquer D, et al. Effectiveness of a preventive
cardiology programme for high CVD risk persistent smokers: the
EUROACTION PLUS varenicline trial. Eur Heart J. 2014;35:1411–1420.
439. Prospective Studies Collaboration, Whitlock G, Lewington S, et al. Body-
mass index and cause-specific mortality in 900 000 adults: collaborative
analyses of 57 prospective studies. Lancet. 2009;373:1083–1096.
440. Herrington WG, Smith M, Bankhead C, et al. Body-mass index and risk of
advanced chronic kidney disease: prospective analyses from a primary
care cohort of 1.4 million adults in England. PLoS One. 2017;12:e0173515.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S303

441. Zhu P, Herrington WG, Haynes R, et al. Conventional and genetic
evidence on the association between adiposity and CKD. J Am Soc
Nephrol. 2021;32:127–137.
442. LOOK AHEAD Research Group. Effect of a long-term behavioural weight
loss intervention on nephropathy in overweight or obese adults with
type 2 diabete s: a secondary analysis of the Look AHEAD randomised
clinical trial. Lancet Diabetes Endocrinol. 2014;2:801–809.
443. Naderi N, Kleine CE, Park C, et al. Obesity paradox in advanced kidney
disease: from bedside to the bench. Prog Cardiovasc Dis. 2018;61:168–
181.
444. Misra A, Jayawardena R, Anoop S. Obesity in South Asia: phenotype,
morbidities, and mitigation. Curr Obes Rep. 2019;8:43–52.
445. World Health Organization. Physical activity.. Accessed May 29, 2023.
https://www.who.int/news-room/fact-sheets/detail/physical-activity
446. Saxena I, Shivankur V, Kumar M. Urinary protein creatinine ratio in
normal zero to three-day-old Indian neonates. J Clin Diagn Res.
2016;10:BC21–BC23.
447. Clark SL, Denburg MR, Furth SL. Physical activity and screen time in
adolescents in the Chronic Kidney Disease in Children (CKiD) cohort.
Pediatr Nephrol. 2016;31:801–808.
448. Adair KE, Bowden RG. Ameliorating chronic kidney disease using a
whole food plant-based diet. Nutrients. 2020;12:1007.
449. Molina P, Gavela E, Vizcaino B, et al. Optimizing diet to slow CKD
progression. Front Med (Lausanne). 2021;8:654250.
450. Cosola C, Rocchetti MT, Sabatino A, et al. Microbiota issue in CKD: how
promising are gut-targeted approaches? J Nephrol. 2019;32:27– 37.
451. Kelly JT, Su G, Zhang L, et al. Modifiable lifestyle factors for primary
prevention of CKD: a systematic review and meta-analysis. J Am Soc
Nephrol. 2021;32:239–253.
452. Kelly JT, Palmer SC, Wai SN, et al. Healthy dietary patterns and risk of
mortality and ESRD in CKD: a meta-analysis of cohort studies. Clin J
Am Soc Nephrol. 2017;12:272–279.
453. Moore LW, Byham-Gray LD, Scott Parrott J, et al. The mean dietary
protein intake at different stages of chronic kidney disease is higher
than current guidelines. Kidney Int. 2013;83:724–732.
454. Torreggiani M, Fois A, Moio MR, et al. Spontaneously low protein intake
in elderly CKD patients: myth or reality? Analysis of baseline protein
intake in a large cohort of patients with advanced CKD. Nutrients.
2021;13:4371.
455. Kalantar-Zadeh K, Kramer HM, Fouque D. High-protein diet is bad for
kidney health: unleashing the taboo. Nephrol Dial Transplant. 2020;35:
1–4.
456. Joint
WHO/FAO/UNU Expert Consultation. Protein and amino acid
requirements in human nutrition. World Health Organization Technical
Report Series; 2007.
457. National Research Council (US) Subcommittee on the Tenth Edition of
the Recommended Dietary Allowances. Definition and applications.
Recommended Dietary Allowances. 10th Edition. National Academies
Press; 1989.
458. Friedman AN. High-protein diets: potential effects on the kidney in renal
health and disease. Am J Kidney Dis. 2004;44:950–962.
459. Hostetter TH, Meyer TW, Rennke HG, et al. Chronic effects of dietary
protein in the rat with intact and reduced renal mass. Kidney Int.
1986;30:509–517.
460. Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy
wasting syndrome in chronic kidney disease: a consensus statement
from the International Society of Renal Nutrition and Metabolism
(ISRNM). J Ren Nutr. 2013;23:77–90.
461. Zahka KG, Manolio TA, Rykiel MJ, et al. Handgrip strength after the
Blalock-Taussig shunt: 14 to 34 year follow-up. Clin Cardiol. 1988;11:
627–629.
462. Malhotra R, Lipworth L, Cavanaugh KL, et al. Protein intake and long-
term change in glomerular filtration rate in the Jackson Heart Study. J
Ren Nutr. 2018;28:245–250.
463. Ko GJ, Rhee CM, Kalantar-Zadeh K, et al. The effects of high-protein diets
on kidney health and longevity. J Am Soc Nephrol. 2020;31:1667–1679.
464. Bernier-Jean A, Prince RL, Lewis JR, et al. Dietary plant and animal
protein intake and decline in estimated glomerular filtration rate
among elderly women: a 10-year longitudinal cohort study. Nephrol
Dial Transplant . 2021;36:1640–1647.
465. Chen X, Wei G, Jalili T, et al. The associations of plant protein intake with
all-cause mortality in CKD. Am J Kidney Dis. 2016;67:423–430.
466. Podadera-Herreros A, Alcala-Diaz JF, Gutierrez-Mariscal FM, et al. Long-
term consumption of a mediterranean diet or a low-fat diet on kidney
function in coronary heart disease patients: the CORDIOPREV
randomized controlled trial. Clin Nutr. 2022;41:552–559.
467. Hu EA, Coresh J, Anderson CAM, et al. Adherence to healthy dietary
patterns and risk of CKD progressi on and all-cause mortality: findings
from the CRIC (Chronic Renal Insufficiency Cohort) study. Am J Kidney
Dis. 2021;77:235–244.
468. Banerjee
T, Crews DC, Tuot DS, et al. Poor accordance to a DASH dietary
pattern is associated with higher risk of ESRD among adults with
moderate chronic kidney disease and hypertension. Kidney Int.
2019;95:1433–1442.
469. Wai SN, Kelly JT, Johnson DW, et al. Dietary patterns and clinical
outcomes in chronic kidney disease: the CKD.QLD nutrition study. J
Ren Nutr. 2017;27:175–182.
470. Gutierrez OM, Muntner P, Rizk DV, et al. Dietary patterns and risk of
death and progression to ESRD in individuals with CKD: a cohort
study. Am J Kidney Dis. 2014;64:204–213.
471. Wong M, Renouf D, Kitchin V, et al. Type of protein and quantity of fruit-
and-vegetable intake on risk of kidney disease progression among
patients with non-dialysis chronic kidney disease: a systematic review
protocol. Accessed September 21, 2023. https://www.crd.york.ac.uk/
prospero/display_record.php?R ecordID¼390548
472. Kontessis P, Jones S, Dodds R, et al. Renal, metabolic and hormonal
responses to ingestion of animal and vegetable proteins. Kidney Int.
1990;38:136–144.
473. Sekiguchi T, KabayamaM, Ryuno H, et al. Association between protein intake
and changes in renal function among Japanese community-dwelling older
people: the SONIC study. Geriatr Gerontol Int. 2022;22:286–291.
474. Sallstrom J, Carlstrom M, Olerud J, et al. High-protein-induced
glomerular hyperfiltration is independent of the tubuloglomerular
feedback mechanism and nitric oxide synthases. Am J Physiol Regu l
Integr Comp Physiol. 2010;299:R1263–R1268.
475. Koppe L, Fouque D. The role for protein restriction in addition to renin-
angiotensin-aldosterone system inhibitors in the management of CKD.
Am J Kidney Dis. 2019;73:248–257.
476. Scharkoff H, Merkel S, Ziemer HM. [Specific and nonspecific tuberculin
sensitivity in school children in East Germany. East German draft
report of a multinational epidemiologic study of the prevalence of
specific and nonspecific tuberculin sensitivity in Europe]. Z Erkr
Atmungsorgane. 1988;170:148–160 [in German].
477. Hahn D, Hodson EM, Fouque D. Low protein diets for non-diabetic adults
withchronickidney disease. Cochrane Database Syst Rev. 2020;10:CD001892.
478. Jiang S, Fang J, Li W. Protein restriction for diabetic kidney disease.
Cochrane Database Syst Rev. 2023;1:CD014906.
479. Garneata L, Stancu A, Dragomir D, et al. Ketoanalogue-supplemented
vegetarian very low-protein diet and CKD progression. J Am Soc
Nephrol. 2016;27:2164– 2176.
480. Bellizzi
V, Signoriello S, Minutolo R, et al. No additional benefitof
prescribing a very low-protein diet in patients with advanced chronic
kidney disease under regular nephrology care: a pragmatic,
randomized, controlled trial. Am J Clin Nutr. 2022;115:1404–1417.
481. Cupisti A, Giannese D, Mori coni D, et al. Nephroprotection by SGLT2i in
CKD patients: may it be modulated by low-protein plant-based Diets?
Front Med (Lausanne). 2020;7:622593.
482. Kalantar-Zadeh K, Beddhu S, Kovesdy CP, et al. Biologically plausible
trends suggesting that a low-protein diet may enhance the effect of
flozination caused by the sodium-glucose cotransporter-2 inhibitor
dapagliflozin on albuminuria. Diabetes Obes Metab. 2021;23:2825 – 2826.
483. Anderson CE, Gilbert RD, Elia M. Basal metabolic rate in children with
chronic kidney disease and healthy control children. Pediatr Nephrol.
2015;30:1995–2001.
484. Uauy RD, Hogg RJ, Brewer ED, et al. Dietary protein and growth in infants
with chronic renal insufficiency: a report from the Southwest Pediatric
Nephrology Study Group and the University of California, San
Francisco. Pediatr Nephrol. 1994;8:45–50.
485. Wingen AM, Fabian-Bach C, Schaefer F, et al. Randomised multicentre
study of a low-protein diet on the progression of chronic renal failure
in children. European Study Group of Nutritional Treatment of Chronic
Renal Failure in Childhood. Lancet. 1997;349:1117–1123.
486. Chaturvedi S, Jones C. Protein restriction for children with chronic renal
failure. Cochrane Database Syst Rev. 2007;4:CD006863.
487. Shaw V, Polderman N, Renken-Terhaerdt J, et al. Energy and protein
requirements for children with CKD stages 2-5 and on dialysis-clinical
practice recommendations from the Pediatric Renal Nutrition
Taskforce. Pediatr Nephrol. 2020;35:519–531.
references www.kidney-international.org
S304
Kidney International (2024) 105 (Suppl 4S), S117–S314

488. KDOQI Work Group. KDOQI clinical practice guideline for nutrition in
children with CKD: 2008 update. Executive summary. Am J Kidney Dis.
2009;53(suppl 2):S11–S104.
489. Volkert D, Beck AM, Cederholm T, et al. ESPEN practical guideline: clinical
nutrition and hydration in geriatrics. Clin Nutr. 2022;41:958–989.
490. Piccoli GB, Cederholm T, Avesani CM, et al. Nutritional status and the risk
of malnutrition in older adults with chronic kidney disease—implications
for low protein intake and nutritional care: a critical review endorsed by
ERN-ERA and ESPEN. Clin Nutr. 2023;42:443–457.
491. World Health Organization. Guideline: sodium intake for adults and
children. 2012. Accessed June 12, 2023. https://iris.who.int/bitstream/
handle/10665/77985/9789241504836_eng.pdf?sequence¼1
492. Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular
events and death. N Engl J Med. 2021;385:1067–1077.
493. McMahon EJ, Campbell KL, Bauer JD, et al. Altered dietary salt intake for
people with chronic kidney disease. Cochrane Database Syst Rev. 2021;6:
CD010070.
494. Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of
reduced dietary sodium and the Dietary Approaches to Stop
Hypertension (DASH) diet. DASH-Sodium Collaborative Research
Group. N Engl J Med. 2001;344:3–10.
495. National Academies of Sciences, Engineering, and Medicine. 2019.
Dietary Reference Intakes for Sodium and Potassium. The National
Academies Press. Accessed June 12, 2023. https://doi.org/10.17226/
25353
496. Simonetti GD, Raio L, Surbek D, et al. Salt sensitivity of children with low
birth weight. Hypertension. 2008;52:625–630.
497. Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual
blood pressure to vascular mortality: a meta-analysis of individual data
for one million adults in 61 prospective studies. Lancet. 2002;360:
1903–1913.
498. SPRINT Research Group, Wright JT Jr, Williamson JD, et al. A randomized
trial of intensive versus standard blood-pressure control. N Engl J Med.
2015;373:2103–2116.
499. Nazarzadeh M, Bidel Z, Canoy D, et al. Blood pressure-lowering
treatment for prevention of major cardiovascular diseases in people
with and without type 2 diabetes: an individual participant-level data
meta-analysis. Lancet Diabetes Endocrinol. 2022;10:645–654.
500. Cheung AK, Whelton PK, Mun tner P, et al. International Consensus on
Standardized Clinic Blood Pressure Measurement—a call to action. Am
J Med. 2023;136:438–
445.
501. McManu
s RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood
pressure, with or without telemonitoring, for titration of
antihypertensive medication (TASMINH4): an unmasked randomised
controlled trial. Lancet. 2018;391:949–959.
502. Janse RJ, Fu EL, Clase CM, et al. Stopping versus continuing renin-
angiotensin-system inhibitors after acute kidney injury and adverse
clinical outcomes: an observational study from routine care data. Clin
Kidney J. 2022;15:1109–1119.
503. Leon SJ, Whitlock R, Rigatto C, et al. Hyperkalemia-related
discontinuation of renin-angiotensin-aldosterone system inhibitors and
clinical outcomes in CKD: a population-based cohort study. Am J
Kidney Dis. 2022;80:164–173.
504. Siew ED, Parr SK, Abdel-Kader K, et al. Renin-angiotensin aldosterone
inhibitor use at hospital discharge among patients with moderate to
severe acute kidney injury and its association with recurrent acute
kidney injury and mortality. Kidney Int. 2021;99:1202–1212.
505. Trevisan M, Fu EL, Xu Y, et al. Stopping mineralocorticoid receptor
antagonists after hyperkalaemia: trial emulation in data from routine
care. Eur J Heart Fail. 2021;23:1698–1707.
506. Xu Y, Fu EL, Trevisan M, et al. Stopping renin-angiotensin system
inhibitors after hyperkalemia and risk of adverse outcomes. Am Heart
J. 2022;243:177–186.
507. Bhandari S, Mehta S, Khwaja A, et al. Renin-angiotensin system inhibition
in advanced chronic kidney disease. N Engl J Med. 2022;387:2021–2032.
508. Fu EL, Evans M, Clase CM, et al. Stopping renin-angiotensin system
inhibitors in patients with advanced CKD and risk of adverse
outcomes: a nationwide study. J Am Soc Nephro l. 2021;32:424–435.
509. Qiao Y, Shin JI, Chen TK, et al. Association between renin-angiotensin
system blockade discontinuation and all-cause mortality among
persons with low estimated glomerular filtration rate. JAMA Intern
Med. 2020;180:718–726.
510. Ku E, Inker LA, Tighiouart H, et al. FR-OR44. Initiation of ACE inhibitor and
ARBs in patients with advanced CKD. J Am Soc Nephrol. 2023;44:40.
511. Nuffield Department of Population Health Renal Studies Group. SGLT
Inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of
diabetes on the effects of sodium glucose co-transporter-2 inhibitors
on kidney outcomes: collaborative meta-analysis of large placebo-
controlled trials. Lancet. 2022;400:1788–1801.
512. StaplinN,RoddickAJ,EmbersonJ,etal.Neteffectsofsodium-glucoseco-
transporter-2 inhibition in different patient groups: a meta-analysis of large
placebo-controlled randomized trials. EClinicalMedicine. 2021;41:101163.
513. Heerspink HJL, Stefansson BV, Correa-Rotter R, et al. Dapagliflozin
in
patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446.
514. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in
type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306.
515. Ferreira JP, Inzucchi SE, Mattheus M, et al. Empagliflozin and uric acid
metabolism in diabetes: a post hoc analysis of the EMPA-REG
OUTCOME trial. Diabetes Obes Metab. 2022;24:135–141.
516. EMPA-KIDNEY Collaborative Group. Design, recruitment, and baseline
characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant.
2022;37:1317–1329.
517. Neuen BL, Oshima M, Agarwal R, et al. Sodium-glucose cotransporter 2
inhibitors and risk of hyperkalemia in people with type 2 diabetes: a
meta-analysis of individual participant data from randomized,
controlled trials. Circulation. 2022;145:1460–1470.
518. Bhatt DL, Szarek M, Pitt B, et al. Sotagliflozin in patients with diabetes
and chronic kidney disease. N Engl J Med. 2021;384:129–139.
519. Willis M, Nilsson A, Kellerborg K, et al. Cost-effectiveness of canagliflozin
added to standard of care for treating diabetic kidney disease (DKD) in
patients with type 2 diabetes mellitus (T2DM) in England: estimates
using the CREDEM-DKD model. Diabetes Ther. 2021;12:313–328.
520. Schechter M, Jongs N, Chertow GM, et al. Effects of dapagliflozin on
hospitalizations in patients with chronic ki dney disease: a post hoc
analysis of DAPA-CKD. Ann Intern Med. 2023;176:59–66.
521. Cherney DZI, Dekkers CCJ, Barbour SJ, et al. Effects of the SGLT2 inhibitor
dapagliflozin on proteinuria in non-diabetic patients with chronic kidney
disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet
Diabetes Endocrinol. 2020;8:582–593.
522. Jhund PS, Ponikowski P, Docherty KF, et al. Dapagliflozin and recurrent
heart failure hospitalizations in heart failure with reduced ejection
fraction: an analysis of DAPA-HF. Circulation. 2021;143:1962–1972.
523. Wanner C, Lachin JM, Inzucchi SE, et al. Empagliflozin and clinical outcomes
in patients with type2 diabetes mellitus, established cardiovascular disease,
and chronic kidney disease. Circulation. 2018;137:119
–12
9.
524. Zannad F, Ferreira JP, Pocock SJ, et al. Cardiac and kidney benefits of
empagliflozin in heart failure across the spectrum of kidney function:
insights from EMPEROR-Reduced. Circulation. 2021;143:310–321.
525. Busse D, Tang W, Scheerer M, et al. Comparison of pharmacokinetics and
the exposure-response relationship of dapagliflozin between
adolescent/young adult and adult patients with type 1 diabetes
mellitus. Br J Clin Pharmacol. 2019;85:1820–1828.
526. Laffel LMB, Tamborlane WV, Yver A, et al. Pharmacokinetic and
pharmacodynamic profile of the sodium-glucose co-transporter-2
inhibitor empagliflozin in young people with type 2 diabetes: a
randomized trial. Diabet Med. 2018;35:1096–1104.
527. Tamborlane WV, Polidori D, Argenti D, et al. Pharmacokinetics and
pharmacodynamics of canagli flozin in pediatric patients with type 2
diabetes. Pediatr Diabetes. 2018;19:649–655.
528. Tirucherai GS, LaCreta F, Ismat FA, et al. Pharmacokinetics and
pharmacodynamics of dapagliflozin in children and adolescents with
type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:678–684.
529. Tamborlane WV, Laffel LM, Shehadeh N, et al. Efficacy and safety of
dapagliflozin in children and young adults with type 2 diabetes: a
prospective, multicentre, randomised, parallel group, phase 3 study.
Lancet Diabetes Endocrinol. 2022;10:341–350.
530. Liu J, Cui J, Fang X, et al. Efficacy and safety of dapagliflozi n in children
with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep.
2022;7:638–641.
531. Currie G, Taylor AH, Fujita T, et al. Effect of mineralocorticoid receptor
antagonists on proteinuria and progression of chronic kidney disease:
a systematic review and meta-analysis. BMC Nephrol. 2016;17:127.
532. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the
diagnosis and treatment of acute and chronic heart failure: developed
by the Task Force for the diagnosis and treatment of acute and
chronic heart failure of the European Society of Cardiology (ESC). With
the special contribution of the Heart Failure Association (HFA) of the
ESC. Eur J Heart Fail. 2022;24:4–131.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S305

533. Bakris GL, Agarwal R, Anker SD, et al. Effect of finerenone on chronic kidney
disease outcomes in type 2 diabete s. N Engl J Med. 2020;383:2219–2229.
534. Pitt B, Filippatos G, Agarwal R, et al. Cardiovascular events with
finerenone in kidney disease and type 2 diabetes. N Engl J Med.
2021;385:2252–2263.
535. Agarwal R, Filippatos G, Pitt B, et al. Cardiovascular and kidney outco mes
with finerenone in patients with type 2 diabetes and chronic kidney
disease: the FIDELITY pooled analysis. Eur Heart J . 2022;43:474–484.
536. Agarwal R, Joseph A, Anker SD, et al. Hyperkalemia risk with finerenone:
results from the FIDELIO-DKD trial. J Am Soc Nephrol. 2022;33:225–237.
537. A Trial to Learn How Well Finerenone Works and How Safe it is in Adult
Participants With Non-diabetic Chronic Kidney Disease (FIND-CKD).
Accessed May 29, 2023. https://clinicaltrials.gov/ct2/show/NCT05047263
537a. Rossing P, Baeres FMM, Bakris G, et al. The rationale, design and baseline
data of FLOW, a kidney outcomes trial with once-weekly semaglutide in
people with type 2 diabetes and chronic kidney disease. Nephrol Dial
Transplant. 2023;38:2041–2051.
538. Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and
kidney outcomes with GLP-1 receptor agonists in patients with type 2
diabetes: a systematic review and meta-analysis of randomised trials.
Lancet Diabetes Endocrinol. 2021;9:653–662.
539. Adamczak M, Surma S. Metabolic acidosis in patients with CKD:
epidemiology, pathogenesis, and treatment. Kidney Dis (Basel). 2021;7:
452–467.
540. Melamed ML, Raphael KL. Metabolic acidosis in CKD: a review of recent
findings. Kidney Med. 2021;3:267–277.
541. Inker LA, Grams ME, Levey AS, et al. Relationship of estimated GFR and
albuminuria to concurrent laboratory abnormalities: an individual
participant data meta-analysis in a Global Consortium. Am J Kidney Dis.
2019;73:206–217.
542. Hultin S, Hood C, Campbell KL, et al. A systematic review and meta-
analysis on effects of bicarbonate therapy on kidney outcomes. Kidney
Int Rep. 2021;6:695–705.
543. BiCarb Study Group. Clinical and cost-effectiveness of oral sodium
bicarbonate therapy for older patients with chronic kidney disease
and low-grade acidosis (BiCARB): a pra gmatic randomised, double-
blind, placebo-controlled trial. BMC Med. 2020;18:91.
544. Wesson DE, Mathur V, Tangri N, et al. Long-term safety and efficacy of
veverimer in patients with metabolic acidosis in chronic kidney
disease: a multicentre, randomised, blinded, placebo-controlled, 40-
week extension. Lancet. 2019;394:396
–406.
545. Mathur
VS, Bushinsky DA, Inker L, et al. Design and population of the
VALOR-CKD study: a multicenter, randomized, double-blind placebo-
controlled trial evaluating the efficacy and safety of veverimer in
slowing progression of chronic kidney disease in patients with
metabolic acidosis. Nephrol Dial Transplant. 2023;38:1448–1458.
546. Carrero JJ, Gonzalez-Ortiz A, Avesani CM, et al. Plant-based diets to
manage the risks and complications of chronic kidney disease. Nat Rev
Nephrol. 2020;16:525–542.
547. Navaneethan SD, Shao J, Buysse J, et al. Effects of treatment of metabolic
acidosis in CKD: a systematic review and meta-analysis. Clin J Am Soc
Nephrol. 2019;14:1011–1020.
548. Goraya N, Munoz-Maldonado Y, Simoni J, et al. Fruit and vegetable
treatment of chronic kidney disease-related metabolic acidosis
reduces cardiovascular risk better than sodium bicarbonate. Am J
Nephrol. 2019;49:438–448.
549. Goraya N, Simoni J, Jo CH, et al. A comparison of treating metabolic
acidosis in CKD stage 4 hypertensive kidney disease with fruits and
vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8:371–381.
550. Goraya N, Simoni J, Jo CH, et al. Treatment of metabolic acidosis in
patients with stage 3 chronic kidney disease with fruits and
vegetables or oral bicarbonate reduces urine angiotensinogen and
preserves glomerular filtration rate. Kidney Int. 2014;86:1031–1038.
551. Noce A, Marrone G, Wilson Jones G, et al. Nutritional approaches for the
management of metabolic acidosis in chronic kidney disease. Nutrients.
2021;13:2534.
552. Brown DD, Roem J, Ng DK, et al. Low serum bicarbonate and CKD
progression in children. Clin J Am Soc Nephrol. 2020;15:755–765.
553. Brown DD, Carroll M, Ng DK, et al. Longitudinal associations between
low serum bicarbonate and linear growth in children with CKD.
Kidney360. 2022;3:666–676.
554. KDOQI: National Kidney Foundation. KDOQI: clinical practice guidelines
for bone metabolism and disease in children with chronic kidney
disease. Am J Kidney Dis. 2005;46:S1–S122.
555. Kovesdy CP, Matsushita K, Sang Y, et al. Serum potassium and adverse
outcomes across the range of kidney function: a CKD Prognosis
Consortium meta-analysis. Eur Heart J. 2018;39:1535–1542.
556. Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and
management of dyskalemia in kidney diseases: conclusions from a
Kidney Disease: Improving Global Outcomes (KDIGO) Controversies
Conference. Kidney Int. 2020;97:42–61.
557. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the
angiotensin-receptor antagonist irbesartan in patients with
nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–
860.
558. Collins
AJ, Pitt B, Reaven N, et al. Association of serum potassium with
all-cause mortality in patients with and without heart failure, chronic
kidney disease, and/or diabetes. Am J Nephrol. 2017;46:213–221.
559. Korgaonkar S, Tilea A, Gillespie BW, et al. Serum potassium and
outcomes in CKD: insights from the RRI-CKD cohort study. Clin J Am
Soc Nephrol. 2010;5:762–769.
560. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and
its significance in chronic kidney disease. Arch Intern Med. 2009;169:
1156–1162.
561. Gasparini A, Evans M, Barany P, et al. Plasma potassium ranges
associated with mortality across stages of chronic kidney disease: the
Stockholm CREAtinine Measurements (SCREAM) project. Nephrol Dial
Transplant. 2019;34:1534–1541.
562. Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality
in acute myocardial infarction. JAMA. 2012;307:157–164.
563. Kolasa KM. Dietary Approaches to Stop Hypertension (DASH) in clinical
practice: a primary care experience. Clin Cardiol. 1999;22:III16–III22.
564. Nakhoul GN, Huang H, Arrigain S, et al. Serum potassium, end-stage
renal disease and mortality in chronic kidney disease. Am J Nephrol.
2015;41:456–463.
565. Allon M, Shanklin N. Effect of albuterol treatment on subsequent dialytic
potassium removal. Am J Kidney Dis. 1995;26:607–613.
566. Foster ES, Jones WJ, Hayslett JP, et al. Role of aldosterone and dietary
potassium in potassium adaptation in the distal colon of the rat.
Gastroenterology. 1985;88:41–46.
567. Gennari FJ, Segal AS. Hyperkalemia: an adaptive response in chronic
renal insufficiency. Kidney Int. 2002;62:1–9.
568. Sandle GI, Gaiger E, Tapster S, et al. Evidence for large intestinal control
of potassium homoeostasis in uraemic patients undergoing long-term
dialysis. Clin Sci (Lond). 1987;73:247– 252.
569. Rastegar A. Clinical methods: the history, physical, and laboratory
examinations. In: Walker HK, Hall WD, Hurst JW, eds. Serum Potassium.
3rd ed. Butterworth; 1990:731.
570. Cooper LB, Savarese G, Carrero JJ, et al. Clinical and research implications
of serum versus plasma potassium measurements. Eur J Heart Fail.
2019;21:536–537.
571. Martin
RS, Panese S, Virginillo M, et al. Increased secretion of potassium
in the rectum of humans with chronic renal failure. Am J Kidney Dis.
1986;8:105–110.
572. St-Jules DE, Goldfarb DS, Sevick MA. Nutrient non-equivalence: does
restricting high-potassium plant foods help to prevent hyperkalemia
in hemodialysis patients? J Ren Nutr. 2016;26:282–287.
573. Wanner C, Fioretto P, Kovesdy CP, et al. Potassium management with
finerenone: practical aspects. Endocrinol Diabetes Metab. 2022;5:e360.
574. Gumz ML, Rabinowitz L. Role of circadian rhythms in potassium
homeostasis. Semin Nephrol. 2013;33:229–236.
575. St-Jules DE, Clegg DJ, Palmer BF, et al. Can novel potassium binders
liberate people with chronic kidney disease from the low-potassium
diet? A cautionary tale. Clin J Am Soc Nephrol. 2022;17:467– 472.
576. Weiner ID, Linas SL, Wingo CS. Chapter 10: Disorders of potassium
metabolism. Comprehensive Clinical Nephrology. Elsevier; 2024:125–136.
577. Pecoits-Filho R, Fliser D, Tu C, et al. Prescription of renin-angiotensin-
aldosterone system inhibitors (RAASi) and its determinants in patients
with advanced CKD under nephrologist care. J Clin Hypertens
(Greenwich). 2019;21:991–1001.
578. Bandak G, Sang Y, Gasparini A, et al. Hyperkalemia after initiating renin-
angiotensin system blockade: the Stockholm Creatinine Measurements
(SCREAM) project. J Am Heart Assoc. 2017;6:e005428.
579. Lazich I, Bakris GL. Prediction and management of hyperkalemia across
the spectrum of chronic kidney disease. Semin Nephrol. 2014;34:333–
339.
580. Trevisan M, de Deco P, Xu H, et al. Incidence, predictors and clinical
management of hyperkalaemia in new users of mineralocorticoid
receptor antagonists. Eur J Heart Fail. 2018;20:1217–1226.
references www.kidney-international.org
S306
Kidney International (2024) 105 (Suppl 4S), S117–S314

581. Bridgeman MB, Shah M, Foote E. Potassium-lowering agents for the
treatment of nonemergent hyperkalemia: pharmacology, dosing and
comparative efficacy. Nephrol Dial Transplant. 2019;34:iii45–iii50.
582. Bushinsky DA, Budden JJ, Kalra PA, et al. Patiromer treatment in patients
with CKD, hyperkalemia, and hyperphosphatemia: a post hoc analysis of
3 clinical trials. Am J Kidney Dis. 2023;82:97–104.
583. Bakris GL, Pitt B, Weir MR, et al. Effect of patiromer on serum potassium
level in patients with hyperkalemia and diabetic kidney disease: the
AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151–161.
584. Roger SD, Lavin PT, Lerma EV, et al. Long-term safety and efficacy of
sodium zirconium cyclosilicate for hyperkalaemia in patients with
mild/moderate versus severe/end-stage chronic kidney disease:
comparative results from an open-label, phase 3 study. Nephrol Dial
Transplant. 2021;36:137–150.
585. Think Kidneys, the Renal Association and the British Society for Heart
Failure. Changes in kidney function and serum potassium during ACE/
ARB/diuretic treatment in primary care. A position statement; 2017.
https://www.thinkkidneys.nhs.uk/aki/news/changes-kidney-function-
serum-potassium-aceiarbdiuretic-treatment-primary-care/
586. UK Kidney Associa tion (UKKA). (2020). Clinical guideline for the
treatment of hyperkalaemia in adults. Accessed May 29, 2023. https://
ukkidney.org/health-professionals/guidelines/treatment-acute-
hyperkalaemia-adults
587. Picard K, Barreto Silva MI, Mager D, et al. Dietary potassium intake and
risk of chronic kidney disease progression in predialysis patients with
chronic kidney disease: a systematic review. Adv Nutr. 2020;11:1002–
1015.
588. Allon M, Dansby L, Shanklin N. Glucose modulation of the disposal of an
acute potassium load in patients with end-stage renal disease. Am J Med.
1993;94:475–482.
589. Ramos CI, Gonzalez-Ortiz A, Espinosa-Cuevas A, et al. Does dietary
potassium intake associate with hyperkalemia in patients with chronic
kidney disease? Nephrol Dial Transplant. 2021;36:2049– 2057.
590. Ikizler TA, Burrowes JD, Byham-Gray LD, et al. KDOQI clinical practice
guideline for nutrition in CKD: 2020 update. Am J Kidney Dis. 2020;76:
S1–S107.
591. Joshi S, McMacken M, Kalantar-Zadeh K. Plant-based diets for kidney
disease: a guide for clinicians. Am J Kidney Dis. 2021;77:287–296.
592. Picard K, Griffiths M, Mager DR, et al. Handouts for low-potassium diets
disproportionately restrict fruits and vegetables. J Ren Nutr. 2021;31:210–
214.
593. Carlisle EJ, Donnelly SM, Ethier JH, et al. Modulation of the secretion of
potassium by accompanying anions in humans. Kidney Int. 1991;39:
1206–1212.
594. Cummings JH, Hill MJ, Jenkins DJ, et al. Changes in fecal composition
and colonic function due to cereal
fiber. Am
J Clin Nutr. 1976;29:1468–
1473.
595. Ceccanti C, Guidi L, D’Alessandro C, et al. Potassium bioaccessibility in
uncooked and cooked plant foods: results from a static in vitro
digestion methodology. Toxins (Basel). 2022;14:668.
596. Parpia AS, L’Abbe M, Goldstein M, et al. The impact of additives on the
phosphorus, potassium, and sodium content of commonly consumed
meat, poultry, and fish products among patients with chronic kidney
disease. J Ren Nutr. 2018;28:83–90.
597. Picard K, Picard C, Mager DR, et al. Potassium content of the American
food supply and implications for the management of hyperkalemia in
dialysis: an analysis of the Branded Product Database. Semin Dial.
Published online July 29, 2021. https://doi.org/10.1111/sdi.13007
598. Sherman RA, Mehta O. Phosphorus and potassium content of enhanced
meat and poultry products: implications for patients who receive
dialysis. Clin J Am Soc Nephrol. 2009;4:1370–1373.
599. de Abreu DBV, Picard K, Klein M, et al. Soaking to reduce potassium and
phosphorus content of foods. J Ren Nutr. 2023;33:165–171.
600. Kurzinski KL, Xu Y, Ng DK, et al. Hyperkalemia in pediatric chronic kidney
disease. Pediatr Nephrol. 2023;38:3083–3090.
601. Desloovere A, Renken-Terhaerdt J, Tuokkola J, et al. The dietary
management of potassium in children with CKD stages 2-5 and on
dialysis-clinical practice recommendations from the Pediatric Renal
Nutrition Taskforce. Pediatr Nephrol. 2021;36:1331–1346.
602. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus,
parathyroid hormone, and calcium and risks of death and
cardiovascular disease in individuals with chronic kidney disease: a
systematic review and meta-analysis. JAMA. 2011;305:1119–1127.
603. Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular
smooth muscle cell calcification. Circ Res. 2000;87:E10–E17.
604. London GM, Guerin AP, Marchais SJ, et al. Arterial media calcification in
end-stage renal disease: impact on all-cause and cardiovascular
mortality. Nephrol Dial Transplant. 2003;18:1731–1740.
605. Liu Z, Su G, Guo X, et al. Dietary interventions for mineral and bone
disorder in people with chronic kidney disease. Cochrane Database
Syst Rev. 2015;2015:CD010350.
606. EVOLVE Trial Investigators, Chertow GM, Block GA, et al. Effect of
cinacalcet on cardiovascular disease in patients undergoing dialysis. N
Engl J Med. 2012;367:2482–2494.
607. FitzGerald JD, Dalbeth N, Mikuls T, et al. 2020 American College of
Rheumatology guideline for the management of gout. Arthritis Care
Res (Hoboken).
2020;72:744–760.
608. Chen-Xu M, Yokose C, Rai SK, et al. Contemporary prevalence of gout
and hyperuricemia in the United States and decadal trends: the
National Health and Nutrition Examination Survey, 2007-2016. Arthritis
Rheumatol. 2019;71:991–999.
609. Sampson AL, Singer RF, Walters GD. Uric acid lowering therapies for
preventing or delaying the progression of chronic kidney disease.
Cochrane Database Syst Rev. 2017;10:CD009460.
610. Yu X, Gu M, Zhu Y, et al. Efficacy of urate-lowering therapy in patients
with chronic kidney disease: a network meta-analysis of randomized
controlled trials. Clin Ther. 2022;44:723–735.e6.
611. Mackenzie IS, Hawkey CJ, Ford I, et al. Allopurinol versus usual care in UK
patients with ischaemic heart disease (ALL-HEART): a multicentre,
prospective, randomised, open-label, blinded-endpoint trial. Lancet.
2022;400:1195–1205.
612. Saag KG, Whelton A, Becker MA, et al. Impact of febuxostat on renal
function in gout patients with moderate-to-severe renal impairment.
Arthritis Rheumatol. 2016;68:2035–2043.
613. Tanaka K, Nakayama M, Kanno M, et al. Renoprotective effects of
febuxostat in hyperuricemic patients with chronic kidney disease: a
parallel-group, randomized, controlled trial. Clin Exp Nephrol. 2015;19:
1044–1053.
614. Wada T, Hosoya T, Honda D, et al. Uric acid-lowering and renoprotective
effects of topiroxostat, a selective xanthine oxidoreductase inhibitor, in
patients with diabetic nephropathy and hyperuricemia: a randomized,
double-blind, placebo-controlled, parallel-group study (UPWARD
study). Clin Exp Nephrol. 2018;22:860–870.
615. Badve SV, Pascoe EM, Tiku A, et al. Effects of allopurinol on the
progression of chronic kidney disease. N Engl J Med. 2020;38 2:2504–
2513.
616. Doria A, Galecki AT, Spino C, et al. Serum urate lowering with allopurinol
and kidney function in type 1 diabetes. N Engl J Med. 2020;382:2493–
2503.
617. Goicoechea M, Garcia de Vinuesa S, Verdalles U, et al. Allopurinol and
progression of CKD and cardiovascular events: long-term follow-up of
a randomized clinical trial. Am J Kidney Dis. 2015;65:543–549.
618. Sircar D, Chatterjee S, Waikhom R, et al. Efficacy of febuxostat for slowing
the GFR decline in patients with CKD and asymptomatic hyperuricemia:
a 6-month, double-blind, randomized, placebo-controlled trial. Am J
Kidney Dis. 2015;66:945–950.
619. Siu YP, Leung KT, Tong MK, et al. Use of allopurinol in slowing the
progression of renal disease through its ability to lower serum uric
acid level. Am J Kidney Dis. 2006;47:51–59.
620. Hill EM, Sky K, Sit M, et al. Does starting allopurinol prolong acute treated
gout? A randomized clinical trial. J Clin Rheumatol. 2015;21:120–
125.
621. Taylor
TH, Mecchella JN, Larson RJ, et al. Initiation of allopurinol at first
medical contact for acute attacks of gout: a randomized clinical trial. Am
J Med. 2012;125:1126–1134.e7.
622. White WB, Saag KG, Becker MA, et al. Cardiovascular safety of febuxostat
or allopurinol in patients with gout. N Engl J Med. 2018;378:1200–1210.
623. Li J, Badve SV, Zhou Z, et al. The effects of canagliflozin on gout in type 2
diabetes: a post-hoc analysis of the CANVAS Program. Lancet Rheumatol.
2019;1:E220–E228.
624. Neogi T, Chen C, Niu J, et al. Alcohol quantity and type on risk of
recurrent gout attacks: an internet-based case-crossover study. Am J
Med. 2014;127:311–318.
625. Ben Salem C, Slim R, Fathallah N, et al. Drug-induced hyperuricaemia
and gout. Rheumatology (Oxford). 2017;56:679–688.
626. Nidorf SM, Fiolet ATL, Mosterd A, et al. Colchicine in patients with
chronic coronary disease. N Engl J Med. 2020;383 :1838 – 1847.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S307

627. Baker M, Perazella MA. NSAIDs in CKD: are they safe? Am J Kidney Dis.
2020;76:546–557.
628. Ralston SH, Capell HA, Sturrock RD. Alcohol and response to treatment
of gout. Br Med J (Clin Res Ed). 1988;296:1641–1642.
629. Stirpe F, Della Corte E, Bonetti E, et al. Fructose-induced hyperuricaemia.
Lancet. 1970;2:1310–1311.
630. Choi JW, Ford ES, Gao X, et al. Sugar-sweetened soft drinks, diet soft
drinks, and serum uric acid level: the Third National Health and
Nutrition Examination Survey. Arthritis Rheum. 2008;59:109–116.
631. Zhang C, Li L, Zhang Y, et al. Recent advances in fructose intake and risk
of hyperuricemia. Biomed Pharmacother. 2020;131:110795.
632. Choi HK, Willett W, Curhan G. Fructose-rich beverages and risk of gout in
women. JAMA. 2010;304:2270–2278.
633. Hui M, Carr A, Cameron S, et al. The British Society for Rheumatology
Guideline for the management of gout. Rheumatology (Oxford).
2017;56:1246.
634. Somkrua R, Eickman EE, Saokaew S, et al. Association of HLA-B*5801
allele and allopurin ol-induced Stevens Johnson syndrome and toxic
epidermal necrolysis: a systematic review and meta-analysis. BMC Med
Genet. 2011;12:118.
635. Jalal DI, Chonchol M, Chen W, et al. Uric acid as a target of therapy in
CKD. Am J Kidney Dis. 2013;61:134–146.
636. Sato Y, Feig DI, Stack AG, et al. The case for uric acid-lowering treatment in
patients with hyperuricaemia and CKD. Nat Rev Nephrol. 2019;15:767–775.
637. Kimura K, Hosoya T, Uchida S, et al. Febuxostat therapy for patients with
stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J
Kidney Dis. 2018;72:798–810.
638. Beddhu S, Filipowicz R, Wang B, et al. A randomized controlled trial of
the effects of febuxostat therapy on adipokines and markers of kidney
fibrosis in asymptomatic hyperuricemic patients with diabetic
nephropathy. Can J Kidney Health Dis. 2016;3:2054358116675343.
639. Hosoya T, Ohno I, Nomura S, et al. Effects of topiroxostat on the serum
urate levels and urinary albumin excretion in hyperuricemic stage 3
chronic kidney disease patients with or without gout. Clin Exp Nephrol.
2014;18:876–884.
640. Jalal DI, Decker E, Perrenoud L, et al. Vascular function and uric acid-
lowering in stage 3 CKD. J Am Soc Nephrol. 2017;28:943–952.
641. Kaiga A, Ishimitsu T, Satonaka H, et al. Therapeutic effects of allopurinol
and topiroxostat in chronic kidney disease patients with hyperuricemia.
Dokkyo J Med Sci
. 2021;48:171–181.
642. Kao
MP, Ang DS, Gandy SJ, et al. Allopurinol benefits left ventricular
mass and endothelial dysfunction in chronic kidney disease. J Am Soc
Nephrol. 2011;22:1382–1389.
643. Mackenzie IS, Ford I, Nuki G, et al. Long-term cardiovascular safety of
febuxostat compared with allopurinol in patients with gout (FAST): a
multicentre, prospective, randomised, open-label, non-inferiority trial.
Lancet. 2020;396:1745–1757.
644. Mukri MNA, Kong W-Y, Mustafar R, et al. Role of febuxostat in retarding
progression of diabetic kidney disease with asymptomatic
hyperuricemia: a 6-months open-label, randomized controlled trial.
EXCLI J. 2018;17:563–575.
645. O’Dell JR, Brophy MT, Pillinger MH, et al. Comparative effectiveness of
allopurinol and febuxostat in gout management. NEJM Evid. 2022;1(3):
10.1056/evidoa2100028.
646. Perrenoud L, Kruse NT, Andrews E, et al. Uric acid lowering and
biomarkers of kidney damage in CKD stage 3: a post hoc analysis of a
randomized clinical trial. Kidney Med. 2020;2:155–161.
647. Saag KG, Becker MA, Whelton A, et al. Efficacy and safety of febuxostat
extended and immediate release in patients with gout and renal
impairment: a phase III placebo-controlled study. Arthritis Rheumatol.
2019;71:143–153.
648. Sezai A, Soma M, Nakata K, et al. Comparison of febuxostat and
allopurinol for hyperuricemia in cardiac surgery patients with chronic
kidney disease (NU-FLASH trial for CKD). J Cardiol . 2015;66:298–303.
649. Sezai A, Unosawa S, Taoka M, et al. Changeover Trial of Febuxostat and
Topiroxostat for Hyperuricemia with Cardiovascular Disease: Sub-
Analysis for Chronic Kidney Disease (TROFEO CKD Trial). Ann Thorac
Cardiovasc Surg. 2020;26:202–208.
650. Sharbaf FG, Assadi F. Effect of allopurinol on the glomerular filtration
rate of children with chronic kidney disease. Pediatr Nephrol. 2018;33:
1405–1409.
651. Wen H, Yongling Z, Shuying Z, et al. Effect of febuxostat on renal
function in patients from South China with CKD3 diabetic
nephropathy. J Bras Nefrol. 2020;42:393–399.
652. Yu H, Liu X, Song Y, et al. Safety and efficacy of benzbromarone and
febuxostat in hyperuricemia patients with chronic kidney disease: a
prospective pilot study. Clin Exp Nephrol. 2018;22:1324–1330.
653. Yang H, Li R, Li Q, et al. Effects of febuxostat on delaying chronic kidney
disease progression: a randomized trial in China. Int Urol Nephrol.
2023;55:1343–1352.
654. Tonelli
M, Muntner P, Lloyd A, et al. Risk of coronary events in people
with chronic kidney disease compared with those with diabetes: a
population-level cohort study. Lancet. 2012;380:807–814.
655. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of
death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:
1296–1305.
656. Park M, Hsu CY, Li Y, et al. Associations between kidney function and
subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23:
1725–1734.
657. Foley RN, Parfrey PS, Kent GM, et al. Long-term evolution of
cardiomyopathy in dialysis patients. Kidney Int. 1998;54:1720–1725.
658. Suzuki T, Agarwal SK, Deo R, et al. Kidney function and sudden cardiac
death in the community: the Atherosclerosis Risk in Communities (ARIC)
study. Am Heart J. 2016;180:46–53.
659. Herzog CA, Littrell K, Arko C, et al. Clinical characteristics of dialysis
patients with acute myocardial infarction in the United States: a
collaborative project of the United States Renal Data System and the
National Registry of Myocardial Infarction. Circulation. 2007;116:1465–
1472.
660. Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in
chronic kidney disease. A clinical update from Kidney Disease:
Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572–586.
661. Twerenbold R, Wildi K, Jaeger C, et al. Optimal cutoff levels of more
sensitive cardiac troponin assays for the early diagnosis of myocardial
infarction in patients with renal dysfunction. Circulation. 2015;131:
2041–2050.
662. Canney M, Tang M, Er L, et al. Glomerular filtration rate-specific cutoffs
can refine the prognostic value of circulating cardiac biomarkers in
advanced chronic kidney disease. Can J Cardiol. 2019;35:1106–1113.
663. Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the
management of acute coronary syndromes in patients presenting
without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–
1367.
664. Kono K, Fujii H, Nakai K, et al. Composition and plaque patterns of
coronary culprit lesions and clinical characteristics of patients with
chronic kidney disease. Kidney Int. 2012;82:344–351.
665. Schiffrin EL, Lipman ML, Mann JF. Chronic kidney disease: effects on the
cardiovascular system. Circulation. 2007;116:85–
97.
666. National
Institute for Health and Care Excellence. Familial
hypercholesterolaemia: identification and management. Clinical
Guideline [CG71]. NICE. 2008.
667. Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for
the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561.
668. Cholesterol Treatment Trialists Collaboration. Effect of statin therapy on
muscle symptoms: an individual participant data meta-analysis of large-
scale, randomised, double-blind trials. Lancet. 2022;400:832– 845.
669. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL
cholesterol with simvastatin plus ezetimibe in patients with chronic
kidney disease (Study of Heart and Renal Protection): a randomised
placebo-controlled trial. Lancet. 2011;377:2181–2192.
670. Reith C, Staplin N, Herrington WG, et al. Effect on non-vascular outcomes
of lowering LDL cholesterol in patients with chronic kidney disease:
results from the Study of Heart and Renal Protection. BMC Nephrol.
2017;18:147.
671. Cholesterol Treatment Trialists Collaboration, Herrington WG,
Emberson J, et al. Impact of renal function on the effects of LDL
cholesterol lowering with statin-based regimens: a meta-analysis of
individual participant data from 28 randomised trials. Lancet Diabetes
Endocrinol. 2016;4:829–839.
672. Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2
diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:
238–248.
673. Fellstrom BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and
cardiovascular events in patients undergoing hemodialysis. N Engl J
Med. 2009;360:1395– 1407.
674. Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA guideline on the
primary prevention of cardiovascular disease: part 1, lifestyle and
behavioral factors. JAMA Cardiol. 2019;4:1043–1044.
references www.kidney-international.org
S308
Kidney International (2024) 105 (Suppl 4S), S117–S314

675. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and
efficacy of the PCSK9 inhibitor evolocumab in patients with and
without diabetes and the effect of evolocumab on glycaemia and risk
of new-onset diabetes: a prespecified analysis of the FOURIER
randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950.
676. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of
alirocumab in reducing lipids and cardiovascular events. N Engl J Med.
2015;372:1489–1499.
677. Charytan DM, Sabatine MS, Pedersen TR, et al. Efficacy and safety of
evolocumab in chronic kidney disease in the FOURIER Trial. J Am Coll
Cardiol. 2019;73:2961–2970.
678. Toth PP, Dwyer JP, Cannon CP, et al. Efficacy and safety of lipid lowering
by alirocumab in chronic kidney disease. Kidney Int. 2018;93:1397–1408.
679. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the
management of dyslipidaemias: lipid modification to reduce
cardiovascular risk. Eur Heart J. 2020;41:111–188.
680. FDA. LeQVIO (inclisiran) injection, for intravenous use [FDA Label]. 2021.
Accessed September 4, 2023. https://www.accessdata.fda.gov/
drugsatfda_docs/label/2021/214 012lbl.pdf
681. Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 dietary guidance to
improve cardiovascular health: a scientific statement from the American
Heart Association. Circulation. 2021;144:e472–e487.
682. Salas-Salvado J, Fernandez-Ballart J, Ros E, et al. Effect of a
Mediterranean diet supplemented with nuts on metabolic syndrome
status: one-year results of the PREDIMED randomized trial. Arch Intern
Med. 2008;168:2449– 2458.
683. Babio N, Toledo E, Estruch R, et al. Mediterranean diets and metabolic
syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:
E649–E657.
684. Delgado-Lista J, Alcala-Diaz JF, Torres-Pena JD, et al. Long-term
secondary prevention of cardiovascular disease with a Mediterranean
diet and a low-fat diet (CORDIOPREV): a randomised controlled trial.
Lancet. 2022;399:1876–1885.
685. Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of
cardiovascular disease with a Mediterranean diet supplemented with
extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34.
685a. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al.
Aspirin in the primary and secondary prevention of vascular disease:
collaborative meta-analysis of individual participant data from
rando
mised trials. Lancet. 2009;373:1849–1860.
686. Natale P, Palmer SC, Saglimbene VM, et al. Antiplatelet agents for
chronic kidney disease. Cochrane Database Syst Rev. 2022;2:CD008834.
687. Bowman L, Mafham M, Stevens W, et al. ASCEND: A Study of
Cardiovascular Events iN Diabetes: characteristics of a randomized trial
of aspirin and of omega-3 fatty acid supplementation in 15,480
people with diabetes. Am Heart J. 2018;198:135–144.
688. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular
events and bleeding in the healthy elderly. N Engl J Med. 2018;379:
1509–1518.
689. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk
of initial vascular events in patients at moderate risk of cardiovascular
disease (ARRIVE): a randomised, double-blind, placebo-controlled trial.
Lancet. 2018;392:1036–1046.
690. Gallagher H, Dumbleton J, Maishman T, et al. Aspirin to target arterial
events in chronic kidney disease (ATTACK): study protocol for a
multicentre, prospective, randomised, open-label, blinded endpoint,
parallel group trial of low-dose aspirin vs. standard care for the
primary prevention of cardiovascular disease in people with chronic
kidney disease. Trials. 2022;23:331.
691. O’Lone E, Viecelli AK, Craig JC, et al. Establishing cor e cardiovascular
outcome measures for trials in hemodialysis: report of an International
Consensus Workshop. Am J Kidney Dis. 2020;76:109–120.
692. Scally B, Emberson JR, Spata E, et al. Effects of gastroprotectant drugs for
the prevention and treatment of peptic ulcer disease and its
complications: a meta-analysis of randomised trials. Lancet
Gastroenterol Hepatol. 2018;3:231–241.
693. Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump
inhibitors based on a large, multi-year, randomized trial of patients
receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682–691.e2.
694. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel
versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet.
1996;348:1329–1339.
695. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in
acute stroke or transient ischemic attack. N Engl J Med. 2016;375:35–43.
696. Agewall S, Cattaneo M, Collet JP, et al. Expert position paper on the use
of proton pump inhibitors in patients with cardiovascular disease and
antithrombotic therapy. Eur Heart J. 2013;34:1708–1713, 1713a–1713b.
697. James S, Budaj A, Aylward P, et al. Ticagrelor versus clopidogrel in acute
coronary syndromes in relation to renal function: results from the
Platelet Inhibition and Patient Outcomes (PLATO) trial. Circulation.
2010;122:1056–1067.
698. Herrington WG, Staplin N. In patients with coronary disease and CKD,
adding an invasive strategy to MT did not improve outcomes. Ann
Intern Med.
2020;173:JC16.
699. Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and
coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol.
2019;74:1823–1838.
700. Charytan DM, Wallentin L, Lagerqvist B, et al. Early angiography in
patients with chronic kidney disease: a collaborative systematic
review. Clin J Am Soc Nephrol. 2009;4:1032–1043.
701. Hastings RS, Hochman JS, Dzavik V, et al. Effect of late revascularization
of a totally occluded coronary artery after myocardial infarction on
mortality rates in patients with renal impairment. Am J Cardiol.
2012;110:954–960.
702. Johnston N, Jernberg T, Lagerqvist B, et al. Early invasive treatment
benefits patients with renal dysfunction in unstable coronary artery
disease. Am Heart J. 2006;152:1052– 1058.
703. Lopes NH, da Silva Paulitsch F, Pereira A, et al. Mild chronic kidney
dysfunction and treatment strategies for stable coronary artery
disease. J Thorac Cardiovasc Surg. 2009;137:1443–1449.
704. Sedlis SP, Jurkovitz CT, Hartigan PM, et al. Optimal medical therapy with
or without percutaneous coronary intervention for patients with stable
coronary artery disease and chronic kidney disease. Am J Cardiol.
2009;104:1647–1653.
705. Vidal-Perez R, Bouzas-Mosquera A, Peteiro J, et al. ISCHEMIA trial: how to
apply the results to clinical practice. World J Cardiol. 2021;13:237–242.
706. Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and
management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477.
707. Maron DJ, Hochman JS, Reynolds HR, et al. Initial invasive or
conservative strategy for stable coronary disease. N Engl J Med.
2020;382:1395–1407.
707a. Bangalore S, Maron DJ, O’Brien SM, et al. Management of coronary
disease in patients with advanced kidney disease. N Engl J Med.
2020;382:1608–1618.
708. James MT, Har BJ, Tyrrell BD, et al. Effect of clinical decision support with
audit and feedback on prevention of acute kidney injury in patients
undergoing coronary angiography: a randomized clinical trial. JAMA .
2022;328:839–849.
709. Hindricks G, Potpara T, Dagres N, et al. Corrigendum to: 2020 ESC
Guidelines for the diagnosis and management of atrial fibrillation
developed in collaboration with the European Association for Cardio-
Thoracic Surgery (EACTS): the Task Force for the diagnosis and
management of atrial fibr
illation of the European Society of Cardiology
(ESC) Developed with the special contribution of the European Heart
Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:507.
710. Turakhia MP, Blankestijn PJ, Carrero JJ, et al. Chronic kidney disease and
arrhythmias: conclusions from a Kidney Disease: Improving Global
Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;39:
2314–2325.
711. Lin WY, Lin YJ, Chung FP, et al. Impact of renal dysfunction on clinical
outcome in patients with low risk of atrial fibrillation. Circ J. 2014;78:
853–858.
712. Szymanski FM, Lip GY, Filipiak KJ, et al. Stroke risk factors beyond the CHA(2)
DS(2)-VASc score: can we improve our identification of "high stroke risk"
patients with atrial fibrillation? Am J Cardiol. 2015;116:1781–1788.
713. de Jong Y, Fu EL, van Diepen M, et al. Validation of risk scores for
ischaemic stroke in atrial fibrillation across the spectrum of kidney
function. Eur Heart J. 2021;42:1476– 1485.
714. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and
safety of new oral anticoagulants with warfarin in patients with atrial
fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–962.
715. Su X, Yan B, Wang L, et al. Oral anticoagulant agents in patients with
atrial fibrillation and CKD: a systematic review and pairwise network
meta-analysis. Am J Kidney Dis. 2021;78:678–689.e1.
716. Bohula E, Giugliano R, Ruff C, et al. Impact of renal function on outcomes
with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. 2016;134:24–36.
717. Chashkina MI, Andreev DA, Kozlovskaya NL, et al. [Safety performance of
rivaroxaban versus warfarin in patients with atrial fibrillation and
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S309

advanced chronic kidney disease]. Kardiologiia. 2020;60:1322 [in
Russian].
718. Fox KA, Piccini JP, Wojdyla D, et al. Prevention of stroke and systemic
embolism with rivaroxaban compared with warfarin in patients with
non-valvular atrial fibrillation and moderate renal impairment. Eur
Heart J. 2011;32:2387–2394.
719. Hijazi Z, Hohnloser SH, Oldgren J, et al. Efficacy and safety of dabigatran
compared with warfarin in patients with atrial fibrillation in relation to
renal function over time—a RE-LY trial analysis. Am Heart J. 2018;198:
169–177.
720. Hori M, Matsumoto M, Tanahashi N, et al. Safety and efficacy of adjusted
dose of rivaroxaban in Japanese patients with non-valvular atrial
fibrillation: subanalysis of J-ROCKET AF for patients with moderate
renal impairment. Circ J. 2013;77:632 – 638.
721. Stanifer J, Pokorney S, Chertow G, et al. Apixaban versus warfarin in
patients with atrial fibrillation and advanced chronic kidney disease.
Circulation. 2020;141:1384–1392.
722. Hijazi Z, Alexander JH, Li Z, et al. Apixaban or vitamin K antagonists and
aspirin or placebo according to kidney function in patients with atrial
fibrillation after acute coronary syndrome or percutaneous coronary
intervention insights from the AUGUSTUS trial. Circulation. 2021;143:
1215–1223.
723. Altawalbeh SM, Alshogran OY, Smith KJ. Cost-utility analysis of apixaban
versus warfarin in atrial fibrillation patients with chronic kidney disease.
Value Health. 2018;21:1365–1372.
724. Heidbuchel H, Verhamme P, Alings M, et al. Updated European Heart
Rhythm Association practical guide on the use of non-vitamin-K
antagonist anticoagulants in patients with non-valvular atrial
fibrillation: executive summary. Eur Heart J. 2017;38:2137–2149.
725. Vondracek SF, Teitelbaum I, Kiser TH. Principles of kidney
pharmacotherapy for the nephrologist: Core Curriculum 2021. Am J
Kidney Dis. 2021;78:442–458.
726. Dorks M, Allers K, Schmiemann G, et al. Inappropriate medication in non-
hospitalized patients with renal insufficiency: a systematic review. JAm
Geriatr Soc. 2017;65:853–862.
727. Long CL, Raebel MA, Price DW, et al. Compliance with dosing guidelines
in patients with chronic kidney disease. Ann Pharmacother. 2004;38:853
–
858.
728. Guirguis
-Blake J, Keppel GA, Holmes J, et al. Prescription of high-risk
medications among patients with chronic kidney disease: a cross-
sectional study from the Washington, Wyoming, Alaska, Montana and
Idaho regio n Practice and Research Network. Fam Pract. 2018;35:589–594.
729. Bosi A, Xu Y, Gasparini A, et al. Use of nephrotoxic medications in adults
with chronic kidney disease in Swedish and US routine care. Clin Kidney
J. 2022;15:442–451.
730. Kimura H, Yoshida S, Takeuchi M, et al. Impact of potentially
inappropriate medications on kidney function in chronic kidney
disease: retrospective cohort study. Nephron. 2023;147:177–184.
731. Clifford KM, Selby AR, Reveles KR, et al. The risk and clinical implications
of antibiotic-associated acute kidney injury: a review of the clinical data
for agents with signals from the Food and Drug Administration’s
Adverse Event Reporting System (FAERS) Database. Antibiotics (Basel).
2022;11:1367.
732. Perazella MA, Rosner MH. Drug-induced acute kidney injury. Clin J Am
Soc Nephrol. 2022;17:1220–1233.
733. Nast CC. Medication-induced interstitial nephritis in the 21st century.
Adv Chronic Kidney Dis. 2017;24:72–79.
734. Klatte DCF, Gasparini A, Xu H, et al. Association between proton pump
inhibitor use and risk of progression of chronic kidney disease.
Gastroenterology. 2017;153:702–710.
735. Brodsky SV, Satoskar A, Chen J, et al. Acute kidney injury during warfarin
therapy associat ed with obstructive tubular red blood cell casts: a report
of 9 cases. Am J Kidney Dis . 2009;54:1121–1126.
736. Golbin L, Vigneau C, Touchard G, et al. Warfarin-related nephropathy
induced by three different vitamin K antagonists: analysis of 13
biopsy-proven cases. Clin Kidney J. 2017;10:381–388.
737. Markowitz GS, Radhakrishnan J, Kambham N, et al. Lithium
nephrotoxicity: a progressive combined glomerular and
tubulointerstitial nephropathy. J Am Soc Nephrol. 2000;11:1439 – 1448.
738. Hall R, Kazancıo
glu R, Thanachayanont T, et al. Drug stewardship for
patients with chronic kidney disease; towards effective, safe, and
sustainable use of medications. Nat Rev Nephrol; in press.
739. Sriperumbuduri S, Hiremath S. The case for cautious consumption: NSAIDs
in chronic kidney disease. Curr Opin Nephrol Hypertens. 2019;28:163–170.
740. Gooch K, Culleton BF, Manns BJ, et al. NSAID use and progression of
chronic kidney disease. Am J Med. 2007;120:280.e281–280.e287.
741. Nelson DA, Marks ES, Deuster PA, et al. Association of nonsteroidal anti-
inflammatory drug prescriptions with kidney disease among active
young and middle-aged adults. JAMA Netw Open.
2019;2:e187896.
742. Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with
the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory
drugs. N Engl J Med. 1994;331:1675–1679.
743. Sandler DP, Burr FR, Weinberg CR. Nonsteroidal anti-inflammatory drugs
and the risk for chronic renal disease. Ann Intern Med. 1991;115:165–172.
744. Novick TK, Surapaneni A, Shin JI, et al. Associations of opioid
prescriptions with death and hospitalization across the spectrum of
estimated GFR. Clin J Am Soc Nephrol. 2019;14:1581–1589.
745. Zhan M, Doerfler RM, Xie D, et al. Association of opioids and nonsteroidal
anti-inflammatory drugs with outcomes in CKD: findings from the CRIC
(Chronic Renal Insufficiency Cohort) study. Am J Kidney Dis. 2020;76:184–
193.
746. Luyckx VA. Nephrotoxicity of alternative medicine practice. Adv Chronic
Kidney Dis. 2012;19:129–141.
747. Yang B, Xie Y, Guo M, et al. Nephrotoxicity and Chinese herbal medicine.
Clin J Am Soc Nephrol. 2018;13:1605–1611.
748. Kilis-Pstrusinska K, Wiela-Hojenska A. Nephrotoxicity of herbal products
in Europe—a review of an underestimated problem. Int J Mol Sci.
2021;22:4132.
749. Steenkamp V, Stewart MJ. Nephrotoxicity associated with exposure to
plant toxins, with particular reference to Africa. Ther Drug Monit.
2005;27:270–277.
750. Koshy KM, Griswold E, Schneeberger EE. Interstitial nephritis in a patient
taking creatine. N Engl J Med. 1999;340:814–815.
751. Thorsteinsdottir B, Grande JP, Garovic VD. Acute renal failure in a young
weight lifter taking multiple food supplements, including creatine
monohydrate. J Ren Nutr. 2006;16:341–345.
752. Xuan BH, Thi TX, Nguyen ST, et al. Ichthyotoxic ARF after fish gallbladder
ingestion: a large case series from Vietnam. Am J Kidney Dis. 2003;41:
220–224.
753. Gabardi S, Munz K, Ulbricht C. A review of dietary supplement-induced
renal dysfunction.
Clin J Am Soc Nephrol.
2007;2:757–765.
754. Perazella MA. Pharmacology behind common drug nephrotoxicities. Clin
J Am Soc Nephrol. 2018;13:1897– 1908.
755. Francis A, Abdul Hafidz MI, Ekrikpo UE, et al. Barriers to accessing
essential medicines for kidney disease in low- and lower middle-
income countries . Kidney Int. 2022;102:969–973.
756. Chang DH, Dumanski SM, Ahmed SB. Female reproductive and
gynecologic considerations in chronic kidney disease: adolescence and
young adulthoo d. Kidney Int Rep. 2022;7:152–164.
757. Kalenga CZ, Dumanski SM, Metcalfe A, et al. The effect of non-oral
hormonal contraceptives on hypertension and blood pressure: a
systematic review and meta-analysis. Physiol Rep. 2022;10:e15267.
758. Tangren J, Bathini L, Jeyakumar N, et al. Pre-pregnancy eGFR and the risk
of adverse maternal and fetal outcomes: a population-based study. JAm
Soc Nephrol. 2023;34:656–667.
759. Dao KH, Bermas BL. Systemic lupus erythematosus management in
pregnancy. Int J Womens Health. 2022;14:199–211.
760. Gotestam Skorpen C, Hoeltzenbein M, Tincani A, et al. The EULAR points
to consider for use of antirheumatic drugs before pregnancy, and during
pregnancy and lactation. Ann Rheum Dis. 2016;75:795–810.
761. Mauvais-Jarvis F, Berthold HK, Campesi I, et al. Sex- and gender-based
pharmacological response to drugs. Pharmacol Rev. 2021;73:730–762.
762. Soldin OP, Mattison DR. Sex differences in pharmacokinetics and
pharmacodynamics. Clin Pharmacokinet. 2009;48:143–157.
763. Burnier M, Pruijm M, Wuerzner G, et al. Drug adherence in chronic
kidney diseases and dialysis. Nephrol Dial Transplant. 2015;30:39–44.
764. Sandhu G, Adattini J, Armstrong GE, et al. International consensus
guideline on anticancer drug dosing in kidney dysfunction. J Clin
Oncol. 2022;40:e13518.
765. Bots SH, Onland-Moret NC, Tulevski II, et al. Heart failure medication
dosage and survival in women and men seen at outpatient clinics.
Heart. 2021;107:1748–1755.
766. Santema BT, Ouwerkerk W, Tromp J, et al. Identifying optimal doses of
heart failure medications in men compared with women: a
prospective, observational, cohort study. Lancet. 2019;394:1254–1263.
767. Miller JA, Cherney DZ, Duncan JA, et al. Gender differences in the renal
response to renin-angiotensin system blockade. J Am Soc Nephrol
.
2006;17:2554–2560.
references www.kidney-international.org
S310
Kidney International (2024) 105 (Suppl 4S), S117–S314

768. Khanal A, Castelino RL, Peterson GM, et al. Dose adjustment guidelines
for medications in patients with renal impairment: how consistent are
drug information sources? Intern Med J. 2014;44:77–85.
769. Butrovich MA, Reaves AC, Heyward J, et al. Inclusion of participants with
CKD and other kidney-related considerations during clinical drug
development: landscape analysis of anticancer agents approved from
2015 to 2019. Clin J Am Soc Nephrol. 2023;18:455–464.
770. U.S. Department of Health and Human Services. Food and Drug
Administration. Center for Drug Evaluation and Research (CDER).
Guidance for Industry Pharmacokinetics in Patients with Impaired Renal
Function—Study Design, Data Analysis, and Impact on Dosing. Draft
Guidance (Revision 2). U.S. Government Printing Office; 2020.
771. European Medicines Agency. Guideline on the evaluation of the
pharmacokinetics of medicinal products in patients with decreased renal
function. 2017. Accessed May 29, 2023. https://www.ema.europa.eu/en/
documents/scientific-guideline/guideline-evaluation-pharmacokinetics-
medicinal-p roducts-patients-decreased-renal-function_en.pdf
772. Titan S, Miao S, Tighiouart H, et al. Performance of indexed and
nonindexed estimated GFR. Am J Kidney Dis. 2020;76:446– 449.
773. Levey AS, Kramer H. Obesity, glomerular hyperfiltration, and the surface
area correction. Am J Kidney Dis. 2010;56:255–258.
774. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute
kidney injury. J Am Soc Nephrol. 2009;20:672–679.
775. Casal MA, Nolin TD, Beumer JH. Estimation of kidney function in
oncology: implications for anticancer drug selection and dosing. Clin J
Am Soc Nephrol. 2019;14:587–595.
776. Claudel SE, Gandhi M, Patel AB, et al. Estimating kidney function in
patients with cancer: a narrative review. Acta Physiol (Oxf). 2023;238:
e13977.
777. Cardone KE, Bacchus S, Assimon MM, et al. Medication-related problems
in CKD. Adv Chronic Kidney Dis. 2010;17:404–412.
778. Roberts DM, Sevastos J, Carland JE, et al. Clinical pharmacokinetics in
kidney disease: application to rational design of dosing regimens. Clin
J Am Soc Nephrol. 2018;13:1254–1263.
779. Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people:
the prescribing cascade. BMJ. 1997;315:1096–1099.
780. Watson KE, Dhaliwal K, McMurtry E, et al. Sick day medication guidance
for people with diabetes, kidney disease, or cardiovascular disease: a
systematic scoping review. Kidney Med. 2022;4:100491.
781.
Duong H, Tesfaye W, Van C, et al. Sick day management in people with
chronic
kidney disease: a scoping review. J Nephrol. 2023;36:1293–1306.
782. Fink JC, Maguire RM, Blakeman T, et al. Medication holds in CKD during
acute volume-depleting illnesses: a randomized controlled trial of a
"sick-day" protocol. Kidney Med. 2022;4:100527.
783. Humphrey TJL, James G, Wittbrodt ET, et al. Adverse clinical outcomes
associated with RAAS inhibitor discontinuation: analysis of over 400
000 patients from the UK Clinical Practice Research Datalink (CPRD).
Clin Kidney J. 2021;14:2203–2212.
784. Morris RL, Ashcroft D, Phipps D, et al. Preventing acute kidney injury: a
qualitative study exploring ’sick day rules’ implementation in primary
care. BMC Fam Pract. 2016;17:91.
785. Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic
agents. Clin J Am Soc Nephrol. 2012;7:1713–1721.
786. Perazella MA, Markowitz GS. Bisphosphonate nephrotoxicity. Kidney Int.
2008;74:1385–1393.
787. Fountoulakis KN, Grunze H, Vieta E, et al. The International College of
Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar
Disorder in Adults (CINP-BD-2017), Part 3: the Clinical Guidelines. Int J
Neuropsychopharmacol. 2017;20:180–195.
788. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for
treating bipolar disorder: revised third edition recommendations from
the British Association for Psychopharmacology. J Psychopharmacol.
2016;30:495–553.
789. Ng F, Mammen OK, Wilting I, et al. The International Society for Bipolar
Disorders (ISBD) consensus guidelines for the safety monitoring of
bipolar disorder treatments. Bipolar Disord. 2009;11:559–595.
790. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA
Guideline for the management of heart failure: executive summary: a
report of the American College of Cardiology/American Heart
Association Joint Committee on Clinical Practice Guidelines. J Am Coll
Cardiol. 2022;79:1757–1780.
791. Martinez YV, Benett I, Lewington AJP, et al. Chronic kidney disease:
summary of updated NICE guidance. BMJ. 2021;374:n1992.
792. Bidulka P, Fu EL, Leyrat C, et al. Stopping renin-angiotensin system
blockers after acute kidney injury and risk of adverse outcomes:
parallel population-based cohort studies in English and Swedish
routine care. BMC Med. 2020;18:195.
793. Bowman C, Lunyera J, Alkon A, et al. A patient safety educational tool for
patients with chronic kidney disease: development and usability study.
JMIR Form Res. 2020;4:e16137.
794. Justad H, Askfors Y, Shemeikka T, et al. Patients’ use and perceptions of a
drug-drug interaction database: a survey of Janusmed interactions.
Pharmacy (Basel). 2021;9:23.
795. Lopez-Vargas PA, Tong A, Howell M, et al. Educational interventions for
patients with CKD: a systematic review . Am J Kidney Dis. 2016;68:353
–
370.
796. Mi
chel G, Levy B, Chauvet MT, et al. Non-mammalian "big"
neurophysins—complete amino acid sequence of a two-domain
MSEL-neurophysin fr om goo se. I nt J Pept Protei n Res. 1990;36:
302–307.
797. Tuot DS, Crowley ST, Katz LA, et al. Usability testing of the kidney score
platform to enhance communication about kidney disease in primary
care settings: qualitative think-aloud study. JMIR Form Res. 2022;6:
e40001.
798. Al Hamarneh YN, Tsuyuki RT, Jones CA, et al. Effectiveness of pharmacist
interventions on cardiovascular risk in patients with CKD: a subgroup
analysis of the randomized controlled RxEACH trial. Am J Kidney Dis.
2018;71:42–51.
799. Al Raiisi F, Stewart D, Fernandez-Llimos F, et al. Clinical pharmacy
practice in the care of chronic kidney disease patients: a systematic
review. Int J Clin Pharm. 2019;41:630–666.
800. Song YK, Jeong S, Han N, et al. Effectiveness of clinical pharmacist
service on drug-related problems and patient outcomes for
hospitalized patients with chronic kidney disease: a randomized
controlled trial. J Clin Med. 2021;10:1788.
801. Awdishu L, Coates CR, Lyddane A, et al. The impact of real-time alerting
on appropriate prescribing in kidney disease: a cluster randomized
controlled trial. J Am Med Inform Assoc. 2016;23:609 – 616.
802. Bhardwaja B, Carroll NM, Raebel MA, et al. Improving prescribing
safety in patients with renal insufficiency in the ambulatory setting:
the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy.
2011;31:346–356.
803. Chertow GM, Lee J, Kuperman GJ, et al. Guided medication dosing for
inpatients with renal insufficiency. JAMA. 2001;286:2839–2844.
804. Erler A, Beyer M, Petersen JJ, et al. How to improve drug dosing for
patients with renal impairment in prima ry care—a cluster-randomized
controlled trial. BMC Fam Pract. 2012;13:91.
805. Sonoda A, Kondo Y, Iwashita Y, et al. In-hospital prescription checking
system for hospitalized patients with decreased glomerular filtration
rate. Kidney360 . 2022;3:1730–1737.
806. Such Diaz A, Saez de la Fuente J, Esteva L, et al. Drug prescribing in
patients with renal impairment optimized by a computer-based, semi-
automated system. Int J Clin Pharm. 2013;35:1170–1177.
807. Goldfarb S, McCullough PA, McDermott J, et al. Contrast-induced acute
kidney injury: specialty-specific protocols for interventional radiology,
diagnostic computed tomography radiology, and interventional
cardiology. Mayo Clin Proc. 2009;84:170–
179.
808. Lee
CD, Hinson J, Davenport MS. Avoiding contrast-enhanced imaging
to prevent contrast-induced acute kidney injury. N Engl J Med.
2022;387:1809–1812.
809. Lee YC, Hsieh CC, Chang TT, et al. Contrast-induced acute kidney injury
among patients with chronic kidney disease undergoing imaging
studies: a meta-analysis. AJR Am J Roentgenol. 2019;213:728–735.
810. Davenport MS, Perazella MA, Yee J, et al. Use of intravenous iodinated
contrast media in patients with kidney disease: consensus statements
from the American College of Radiology and the National Kidney
Foundation. Radiology. 2020;294:660–668.
811. Weisbord SD, Mor MK, Hochheiser H, et al. Utilization and outcomes of
clinically indicated invasive cardiac care in veterans with acute coronary
syndrome and chronic kidney disease. J Am Soc Nephrol. 2023;34:694–
705.
812. Chertow GM, Normand SL, McNeil BJ. “Renalism”: inappropriately low
rates of coronary angiography in elderly individuals with renal
insufficiency. J Am Soc Nephrol. 2004;15:2462–2468.
813. Cashion W, Weisbord SD. Radiographic contrast media and the kidney.
Clin J Am Soc Nephrol. 2022;17:1234–1242.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S311

814. Schonenberger E, Martus P, Bosserdt M, et al. Kidney injury after
intravenous versus intra-arterial contrast agent in patients suspected
of having coronary artery disease: a randomized trial. Radiology.
2019;292:664–672.
815. Prasad A, Palevsky PM, Bansal S, et al. Management of patients with
kidney disease in need of cardiovascular catheterization: a scientific
workshop cosponsored by the National Kidney Foundation and the
Society for Cardiovascular Angiography and Interventions. J Soc
Cardiovasc Angiogr Interv. 2022;1:100445.
816. Jurado-Roman A, Hernandez-Hernandez F, Garcia-Tejada J, et al. Role of
hydration in contrast-induced nephropathy in patients who underwent
primary percutaneous coronary intervention. Am J Cardio l. 2015;115:
1174–1178.
817. Luo Y, Wang X, Ye Z, et al. Remedial hydration reduces the inciden ce of
contrast-induced nephropathy and short-term adverse events in
patients with ST-segment elevation myocardial infarction: a single-
center, randomized trial. Intern Med. 2014;53:2265–2272.
818. Macdonald DB, Hurrell CD, Costa AF, et al. Canadian Association of
Radiologists Guidance on contrast-associated acute kidney injury. Can
J Kidney Health Dis. 2022;9:20543581221097455.
819. Sebastia C, Paez-Carpio A, Guillen E, et al. Oral hydration as a safe
prophylactic measure to prevent post-contrast acute kidney injury in
oncologic patients with chronic kidney disease (IIIb) referred for
contrast-enhanced computed tomography: subanalysis of the
oncological group of the NICIR study. Support Care Cancer. 2022;30:
1879–1887.
820. Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a
review of known and proposed mechanisms. Biometals. 2016;29:365–
376.
821. Endrikat J, Dohanish S, Schleyer N, et al. 10 years of nephrogenic
systemic fibrosis: a comprehensive analysis of nephrogenic systemic
fibrosis reports received by a pharmaceutical company from 2006 to
2016. Invest Radiol. 2018;53:541–550.
822. Khawaja AZ, Cassidy DB, Al Shakarchi J, et al. Revisiting the risks of MRI
with Gadolinium based contrast agents-review of literature and
guidelines. Insights Imaging. 2015;6:553–558.
823. Weinreb JC, Rodby RA, Yee J, et al. Use of intravenous gadolinium-based
contrast media in patients with kidney disease: consensus statements
from the American College of Radiology and the National Kidney
Foundation. Radiology. 2021;298:28–35.
824. Boyd AS, Zic JA, Abraham JL. Gadolinium deposition in nephrogenic
fibrosing dermopathy. J Am Acad Dermatol. 2007;56:27–30.
825. Schieda N, Maralani PJ, Hurrell C, et al. Updated clinical practice
guideline on use of gadolinium-based contrast agents in kidney
disease issued by the Canadian Association of Radiologists. Can Assoc
Radiol J. 2019;70:226–232.
826. ACR Committee on Drugs and Contrast Media. ACR manual on contrast
media.
American College of Radiology; 2023. Accessed May 29, 2023.
https://www.acr.org/-/media/acr/ files/clinical-resources/contrast_media.
pdf
827. Wang PI, Chong ST, Kielar AZ, et al. Imaging of pregnant and lactating
patients: part 1, evidence-based review and recommendations. AJR
Am J Roentgenol. 2012;198:778–784.
828. Nardone B, Saddleton E, Laumann AE, et al. Pediatric nephrogenic
systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol.
2014;44:173–180.
829. Bhachu HK, Cockwell P, Subramanian A, et al. Impact of using risk-based
stratification on referral of patients with chronic kidney disease from
primary care to specialist care in the United Kingdom. Kidney Int Rep.
2021;6:2189–2199.
830. Ramspek CL, Boekee R, Evans M, et al. Predicting kidney failure,
cardiovascular disease and death in advanced CKD patients. Kidney Int
Rep. 2022;7:2 230–2241.
831. Navaneethan SD, Aloudat S, Singh S. A systematic review of patient and
health system characteristics associated with late referral in chronic
kidney disease. BMC Nephrol. 2008;9:3.
832. Smart NA, Dieberg G, Ladhani M, et al. Early referral to specialist
nephrology services for preventing the progression to end-stage
kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
833. Lonnemann G, Duttlinger J, Hohmann D, et al. Timely referral to
outpatient nephrology care slows progression and reduces treatment
costs of chronic kidney diseases. Kidney Int Rep. 2017;2:142–151.
834. Oliva-Damaso N, Oliva-Damaso E, Rivas-Ruiz F, et al. Impact of a phone
app on nephrology referral. Clin Kidney J. 2019;12:427–432.
835. Rhee CM, Edwards D, Ahdoot RS, et al. Living well with kidney disease
and effective symptom management: Consensus Conference
Proceedings. Kidney Int Rep. 2022;7:1951–1963.
836. Kalantar-Zadeh K, Lockwood MB, Rhee CM, et al. Patient-centred
approaches for the management of unpleasant symptoms in kidney
disease. Nat Rev Nephrol. 2022;18:185–198.
837. Morton RL, Tong A, Howard K, et al. The views of patients and carers in
treatment decision making for chronic kidney disease: systematic review
and thematic synthesis of qualitative studies. BMJ. 2010;340:c112.
838. Davison SN, Levin A, Moss AH, et al. Executive summary of the KDIGO
Controversies Conference on Supportive Care in Chronic Kidney
Disease: developing a roadmap to improving quality care. Kidney Int.
2015;88:447–459.
839. Fletcher BR, Damery S, Aiyegbusi OL, et al. Symptom burden and health-
related quality of life in chronic kidney disease: a global systematic
review and meta-analysis. PLoS
Med. 2022;19:e1003954.
840. Metzger M, Abdel-Rahman EM, Boykin H, et al. A narrative review of
management strategies for common symptoms in advanced CKD.
Kidney Int Rep. 2021;6:894–904.
841. Verberne WR, Das-Gupta Z, Allegretti AS, et al. Development of an
international standard set of value-based outcome measures for
patients with chronic kidney disease: a report of the International
Consortium for Health Outcomes Measurement (ICHOM) CKD Working
Group. Am J Kidney Dis. 2019;73:372–384.
842. Chen SS, Unruh M, Williams M. In quality we trust; but quality of life or
quality of care? Semin Dial. 2016;29:103–110.
843. Kliger AS. Quality measures for dialysis: time for a balanced scorecard.
Clin J Am Soc Nephrol. 2016;11:363–368.
844. Selewski DT, Massengill SF, Troost JP, et al. Gaining the Patient Reported
Outcomes Measurement Information System (PROMIS) perspective in
chronic kidney disease: a Midwest Pediatric Nephrology Consortium
study. Pediatr Nephrol. 2014;29:2347–2356.
845. van der Willik EM, van Breda F, van Jaarsveld BC, et al. Validity and reliability of
Patient-Reported Outcomes Measurement Information System (PROMIS)
using Computerized Adaptive Testing (CAT) in patients with advanced
chronic kidney disease. Nephrol Dial Transplant. 2022;38:1158–1169 .
846. Tang E, Ekundayo O, Peipert JD, et al. Validation of the Patient-Reported
Outcomes Measurement Information System (PROMIS)-57 and -29 item
short forms among kidney transplant recipients. Qual Life Res. 2019;28:
815–827.
847. Harrison TG, Skrtic M, Verdin NE, et al. Improving sexual function in people
with chronic kidney disease: a narrative review of an unmet need in
nephrology research. Can J Kidney Health Dis. 2020;7:2054358120952202.
848. Davison SN, Rathwell S, Ghosh S, et al. The prevalence and severity of
chronic pain in patients with chronic kidney disease: a systematic
review and meta-analysis. Can J Kidney Health Dis. 2021;8:
2054358121993995.
849. Koncicki HM, Unruh M, Schell JO. Pain management in CKD: a guide for
nephrology providers. Am J Kidney Dis. 2017;69:451–460.
850. Davison SN, Koncicki H, Brennan F. Pain in chronic kidney disease: a
scoping review. Semin Dial. 2014;27:188–204.
851. Koch BC, Nagtegaal JE, Hagen EC, et al. The effects of melatonin on
sleep-wake rhythm of daytime haemodialysis patie nts: a randomized,
placebo-controlled, cross-over study (EMSCAP study). Br J Clin
Pharmacol. 2009;67:68–75.
852. Biyik Z, Solak Y, Atalay H, et al. Gabapentin versus pregabalin in
improving sleep quality and depression in hemodialysis patients with
peripheral neuropathy: a randomized prospective crossover trial. Int
Urol Nephrol. 2013;45:831–
837.
853. Pagel
JF, Pandi-Perumal SR, Monti JM. Treating insomnia with
medications. Sleep Sci Pract. 2018;2:5.
854. De Santo RM, Bartiromo M, Cesare MC, et al. Sleeping disorders in early
chronic kidney disease. Semin Nephrol. 2006;26:64–67.
855. Cheikh Hassan HI, Brennan F, Collett G, et al. Efficacy and safety of
gabapentin for uremic pruritus and restless legs syndrome in
conservatively managed patients with chronic kidney disease. J Pain
Symptom Manage. 2015;49:782–789.
856. Giannaki CD, Sakkas GK, Karatzaferi C, et al. Effect of exercise training
and dopamine agonists in patients with uremic restless legs
syndrome: a six-month randomized, partially double-blind, placebo-
controlled comparative study. BMC Nephrol. 2013;14:194.
857. Wetter TC, Trenkwalder C, Stiasny K, et al. [Treatment of idiopathic and
uremic restless legs syndrome with L-dopa–a double-blind cross-over
study]. Wien Med Wochenschr. 1995;145:525–527 [in German].
references www.kidney-international.org
S312
Kidney International (2024) 105 (Suppl 4S), S117–S314

858. Lu PH, Chung CH, Chuo HE, et al. Efficacy of acupoint stimulation as a
treatment for uremic pruritus: a systematic review and meta-analysis.
Front Med (Lausanne). 2022;9:1036072.
859. Simonsen E, Komenda P, Lerner B, et al. Treatment of uremic pruritus: a
systematic review. Am J Kidney Dis. 2017;70:638–655.
860. Mettang T, Kremer AE. Uremic pruritus. Kidney Int. 2015;87:685–691.
861. Manenti L, Tansinda P, Vaglio A. Uraemic pruritus: clinical characteristics,
pathophysiology and treatment. Drugs. 2009;69:251–263.
862. Scherer JS, Combs SA, Brennan F. Sleep disorders, restless legs
syndrome, and uremic pruritus: diagnosis and treatment of common
symptoms in dialysis patients. Am J Kidney Dis. 2017;69:117–128.
863. Ho C, Martinusen D, Lo C. A review of cannabis in chronic kidney disease
symptom management. Can J Kidney Health Dis. 2019;6:
2054358119828391.
864. Barcellos FC, Santos IS, Umpierre D, et al. Effects of exercise in the whole
spectrum of chronic kidney disease: a systematic review. Clin Kidney J.
2015;8:753–765.
865. Kim KH, Lee MS, Kim TH, et al. Acupuncture and related interventions for
symptoms of chronic kidney disease. Cochrane Database Syst Rev.
2016;2016:CD009440.
866. Baghdady NT, Banik S, Swartz SA, et al. Psychotropic drugs and renal
failure: translating the evidence for clinical practice. Adv Ther. 2009;26:
404–424.
867. Cohen LM, Tessier EG, Germain MJ, et al. Update on psychotropic
medication use in renal disease. Psychosomatics. 2004;45:34–48.
868. Constantino JL, Fonseca VA. Pharmacokinetics of antidepressants in
patients undergoing hemodialysis: a narrative literature review. Braz J
Psychiatry. 2019;41:441–446.
869. Nagler EV, Webster AC, Vanholder R, et al. Antidepressants for
depression in stage 3-5 chronic kidney disease: a systematic review of
pharmacokinetics, efficacy and safety with recommendations by
European Renal Best Practice (ERBP). Nephrol Dial Transplant. 2012;27:
3736–3745.
870. Duarte PS, Miyazaki MC, Blay SL, et al. Cognitive-behavioral group
therapy is an effective treatment for major depression in hemodialysis
patients. Kidney Int. 2009;76:414–421.
871. Tentori F, Elder SJ, Thumma J, et al. Physical exercise among participants
in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates
and associated outcomes. Nephrol Dial Transplant. 2010;25:3050–
3062.
872. Carrero
JJ, Thomas F, Nagy K, et al. Global prevalence of protein-energy
wasting in kidney disease: a meta-analysis of contemporary
observational studies from the International Society of Renal Nutrition
and Metabolism. J Ren Nutr. 2018;28:380–392.
873. Iorember FM. Malnutrition in chronic kidney disease. Front Pediatr.
2018;6:161.
874. Wright M, Southcott E, MacLaughlin H, et al. Clinical practice guideline
on undernutrition in chronic kidney disease. BMC Nephrol. 2019;20:370.
875. Windahl K, Faxen Irving G, Almquist T, et al. Prevalence and risk of
protein-energy wasting assessed by subjective global assessment in
older adults with advanced chronic kidney disease: results from the
EQUAL study. J Ren Nutr. 2018;28:165–174.
876. Lim SL, Lin XH, Daniels L. Seven-point subjective global assessment is
more time sensitive than conventional subjective global assessment in
detecting nutrition changes. JPEN J Parenter Enteral Nutr. 2016;40:966–
972.
877. Graterol Torres F, Molina M, Soler-Majoral J, et al. Evolving concepts on
inflammatory biomarkers and malnutrition in chronic kidney disease.
Nutrients. 2022;14:4297.
878. Pawlaczyk W, Rogowski L, Kowalska J, et al. Assessment of the nutritional
status and quality of life in chronic kidney disease and kidney transplant
patients: a comparative analysis. Nutrients. 2022;14:4814.
879. Epping-Jordan JE, Pruitt SD, Bengoa R, et al. Improving the quality of
health care for chronic conditions. Qual Saf Health Care. 2004;13:299–
305.
880. Nicoll R, Robertson L, Gemmell E, et al. Models of care for chronic kidney
disease: a systematic review. Nephrology (Carlton). 2018;23:389–396.
881. Collister D, Pyne L, Cunningham J, et al. Multidisciplinary chronic kidney
disease clinic practices: a scoping review. Can J Kidney Health Dis. 2019;6:
2054358119882667.
882. Nkunu V, Wiebe N, Bello A, et al. Update on existing care models for
chronic kidney disease in low- and middle-income countries: a
systematic review. Can J Kidney Health Dis. 2022;9:20543581221077505.
883. Thanachayanont T, Chanpitakkul M, Hengtrakulvenit J, et al.
Effectiveness of integrated care on delaying chronic kidney disease
progression in rural communities of Thailand (ESCORT-2) trials.
Nephrology (Carlton). 2021;26:333–340.
884. Lewis R. Remote monitoring of chronic kidney disease. The Clinical Services
Journal.. Accessed May 29, 2023. https://www.clinicalservicesjournal.com/
story/34805/remote-monitoring-of-chronic-kidney-disease
885. Wieringa FP, Broers NJH, Kooman JP, et al. Wearable sensors: can they
benefit patients with chronic kidney disease? Expert Rev Med Devices.
2017;14:505–519.
886. Ong SW, Wong JV, Auguste BL, et al. Design and development of a
digital counseling program for chronic kidney disease. Can J Kidney
Health Dis. 2022;9:20543581221103683.
887. Morony S, Flynn M, McCaffery KJ, et al. Readability of written materials
for CKD patients: a systematic review. Am
J Kidney Dis. 2015;65:842–850.
888. Tuot DS, Davis E, Velasquez A, et al. Assessment of printed patient-
educational materials for chronic kidney disease. Am J Nephrol.
2013;38:184–194.
889. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-
management system into usual care of advanced CKD. Clin J Am Soc
Nephrol. 2016;11:1054– 1062.
890. Easom AM, Shukla AM, Rotaru D, et al. Home run-results of a chronic
kidney disease Telemedicine Patient Education Study. Clin Kidney J.
2020;13:867–872.
891. Young A, Orchanian-Cheff A, Chan CT, et al. Video-based telemedicine
for kidney disease care: a scoping review. Clin J Am Soc Nephrol.
2021;16:1813–1823.
892. LaRosa C, Glah C, Baluarte HJ, et al. Solid-org an transplantation in
childhood: transitioning to adult health care. Pediatrics. 2011;127:742–753.
893. Singh SP, Anderson B, Liabo K, et al. Supporting young people in their
transition to adults’ services: summary of NICE guidance. BMJ.
2016;353:i2225.
894. Watson AR, Harden P, Ferris M, et al. Transition from pediatric to adult
renal services: a consensus statement by the International Society of
Nephrology (ISN) and the International Pediatric Nephrology
Association (IPNA). Pediatr Nephrol. 2011;26:1753–1757.
895. Francis A, Johnson DW, Craig JC, et al. Moving on: transitioning young
people with chronic kidney disease to adult care. Pediatr Nephrol .
2018;33:973–983.
896. Ferris ME, Harward DH, Bickford K, et al. A clinical tool to measure the
components of health-care transition from pediatric care to adult care:
the UNC TRxANSITION scale. Renal Failure. 2012;34:744–753.
897. Gilleland J, Amaral S, Mee L, et al. Getting ready to leave: transition
readiness in adolescent kidney transplant recipients. J Pediatr Psychol.
2012;37:85–96.
898. Paone MC, Wigle M, Saewyc E. The ON TRAC model for transitional care
of adolescents. Prog Transplant. 2006;16:291–302.
899. Sawicki GS, Lukens-Bull K, Yin X, et al. Measuring the transition readiness
of youth with special healthcare needs: validation of the TRAQ—
Transition Readiness Assessment Questionnaire. J Pediatr Psychol.
2011;36:160–171.
900.
Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated
paediatri
c to adult clinical service for young adults with kidney failure.
BMJ. 2012;344:e3718.
901. Prestidge C, Romann A, Djurdjev O, et al. Utility and cost of a renal
transplant transition clinic. Pediatr Nephrol. 2012;27:295–302.
902. Pape L, Lammermuhle J, Oldhafer M, et al. Different models of transition
to adult care after pediatric kidney transplantation: a comparative study.
Pediatr Transplant. 2013;17:518–524.
903. Foster B. Heightened graft failure risk during emerging adulthood and
transition to adult care. Pediatr Nephrol. 2015;30:567–576.
904. Foster B, Bell L. Improving the transition to adult care for young people
with chronic kidney disease. Curr Pediatr Rep. 2015;3:62–70.
905. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of
early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619.
906. Harris A, Cooper BA, Li JJ, et al. Cost-effectiveness of initiating dialysis
early: a randomized controlled trial. Am J Kidney Dis. 2011;57:707–715.
907. Whalley GA, Marwick TH, Doughty RN, et al. Effect of early initiation of
dialysis on cardiac structure and function: results from the echo
substudy of the IDEAL trial. Am J Kidney Dis. 2013;61:262–270.
908. Fu EL, Evans M, Carrero JJ, et al. Timing of dialysis initiation to reduce
mortality and cardiovascular events in advanced chronic kidney
disease: nationwide cohort study. BMJ. 2021;375:e066306.
909. Nacak H, Bolignano D, Van Diepen M, et al. Timing of start of dialysis in
diabetes mellitus patients: a systematic literature review. Nephrol Dial
Transplant. 2016;31:306–316.
www.kidney-international.org references
Kidney International (2024) 105 (Suppl 4S), S117–S314 S313

910. Rosansky SJ, Eggers P, Jackson K, et al. Early start of hemodialysis may be
harmful. Arch Intern Med. 2011;171:396–403.
911. Preka E, Bonthuis M, Harambat J, et al. Association between timing of
dialysis initiation and clinical outcomes in the paediatric population:
an ESPN/ERA-EDTA registry study. Nephrol Dial Transplant. 2019;34:
1932–1940.
912. Winnicki E, Johansen KL, Cabana MD, et al. Higher eGFR at dialysis
initiation is not associated with a survival benefit in children. J Am Soc
Nephrol. 2019;30:1505–1513.
913. Larkins NG, Lim W, Goh C, et al. Timing of kidney replacement therapy
among children and young adults. Clin J Am Soc Nephrol. 2023;18:1041–
1050.
914. Okuda Y, Soohoo M, Tang Y, et al. Estimated GFR at dialysis initiation and
mortality in children and adolescents. Am J Kidney Dis. 2019;73:797–805.
915. Hole B, Hemmelgarn B, Brown E, et al. Supportive care for end-stage
kidney disease: an integral part of kidney services across a range of
income settings around the world. Kidney Int Suppl (2011). 2020;10:
e86–e94.
916. Institute of Medicine. Finding What Works in Health Care: Standards for
Systematic Reviews. The National Academies Press ; 2011.
917. Institute of Medicine (US). Committee on standards for develop ing
trustworthy clinical practice guidelines. In: Graham R, Mancher M,
Miller Wolman DW, et al., eds. Clinical Practice Guidelines We Can Trust.
National Academi es Press; 2011.
918. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline
development, reporting and evaluation in health care. J Clin Epidemiol.
2010;63:1308–1311.
919. Higgins JPT, Thomas J, Chandler J, et al., eds. Cochrane Handbook for
Systematic Reviews of Interventions. 2nd ed. Wiley; 2019.
920. Poggio ED, McClelland RL, Blank KN, et al. Systematic review and meta-
analysis of native kidney biopsy complications. Clin J Am Soc Nephrol.
2020;15:1595–1602.
921. National Institute for Health and Care Excellence (NICE). Evidence
review for the accuracy of albumin:creatinine ratio versus protein
creatinine ratio measurements to quantify proteinuria in children and
young people with CKD: Chronic kidney disease: Evidence review B.
London: NICE; 2021.
922. Corbett M, Duarte A, Llewellyn A, et al. Point-of-care creatinine tests to
assess kidney function for outpatients requiring contrast-enhanced CT
imaging: systematic reviews and economic evaluation. Health Technol
Assess. 2020;24:1– 248.
923. Chung EY, Ruospo M, Natale P, et al. Aldosterone antagonists in addition
to renin angiotensin system antagonists for preventing the progression
of chronic kidney disease. Cochrane Database Syst Rev
. 2020;10:
Cd00700
4.
924. Kamdar A, Sykes R, Morrow A, et al. Cardiovascular outcomes of glucose
lowering therapy in chronic kidney disease patients: a systematic review
with meta-analysis. Rev Cardiovasc Med. 2021;22:1479–1490.
925. Pallikadavath S, Ashton L, Brunskill NJ, et al. Aspirin for the primary
prevention of cardiovascular disease in individuals with chronic kidney
disease: a systematic review and meta-analysis. Eur J Prev Cardiol.
2022;28:1953–1960.
926. Kimachi M, Furukawa TA, Kimachi K, et al. Direct oral anticoagulants
versus warfarin for preventing stroke and systemic embolic events
among atrial fibrillation patients with chronic kidney disease. Cochrane
Database Syst Rev. 2017;11:CD011373.
927. Sterne JAC, Savovi
c J, Page MJ, et al. RoB 2: a revised tool for assessing
risk of bias in randomised trials. BMJ. 2019;366:l4898.
928. Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for
the quality assessment of diagnostic accuracy studies. Ann Intern Med.
2011;155:529–536.
929. Whiting P, Savovi
c J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias
in systematic reviews was developed. J Clin Epidemiol. 2016;69:225–234.
930. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials .
1986;7:177–188.
931. Freeman MF, Tukey J. Transformations related to the angular and the
square root. Ann Math Stat. 1950;21:601–611.
932. Newcombe RG. Two-sided confidence intervals for the single proportion:
comparison of seven methods. Stat Med. 1998;17:857–872.
933. Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in
meta-analyses. BMJ. 2003;327:557–560.
934. Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an
overall rating of confidence in effect estimates for a single outcome
and for all outcomes. J Clin Epidemiol. 2013;66:151–157.
935. Schünemann H, Bro
_
zek J, Guyatt G, et al. GRADE handbook for grading
quality of evidence and strength of recommendations. Updated October
2013. The GRADE Working Group; 2013.
936. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the
quality of evidence
–impreci
sion. J Clin Epidemiol. 2011;64:1283–1293.
937. Brunetti M, Shemilt I, Pregno S, et al. GRADE guidelines: 10. Considering
resource use and rating the quality of economic evidence. J Clin
Epidemiol. 2013;66:140–150.
references www.kidney-international.org
S314
Kidney International (2024) 105 (Suppl 4S), S117–S314

