
Functional Groups
Functional
Groups
Functional
g
rou
p
- collection of atoms at a site
gp
within a molecule with a common bonding pattern
The group reacts in a typical way, generally
independent of the rest of the molecule
independent
of
the
rest
of
the
molecule
For example, the double bonds in simple and
complex alkenes react with bromine in the same way
(See Figure 3 1)
(See
Figure
3
.
1)

Types of Functional Groups: Multiple
b
bd
Car
b
on
–
Car
b
on Bon
d
s
A
lkanes have onl
y
y
C-C and C-H single
bonds
Alkenes
have a C
-
C
Alkenes
have
a
C
C
double bond
Alkynes have a C-C
triple bond
triple
bond
Arenes have special
bonds that are
td
represen
t
e
d
as
alternating single
and double C-C
bdi i
b
on
d
s
i
n a s
i
x-
membered ring

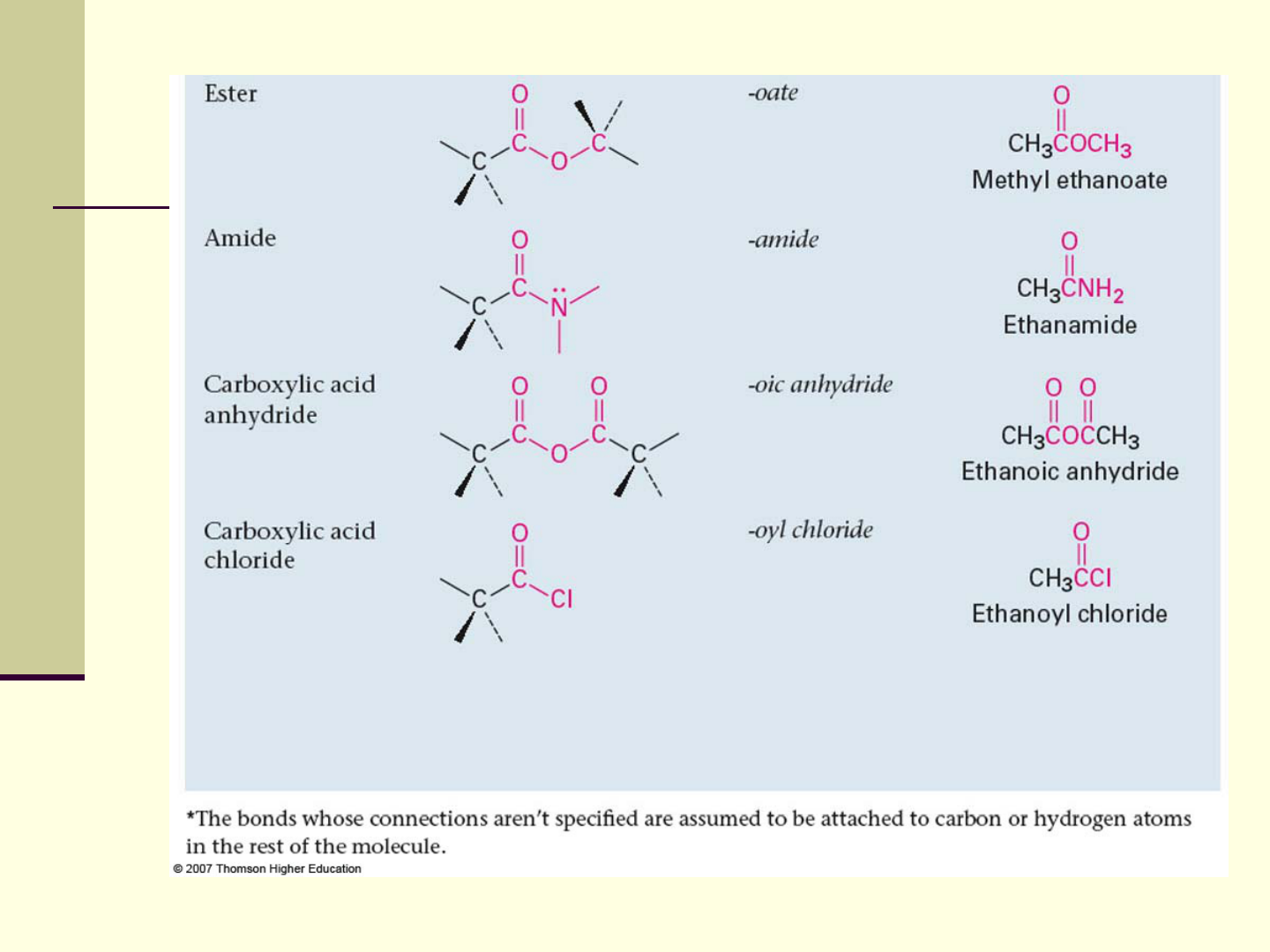
Functional Groups with Carbon Singly
dd l i
Bon
d
e
d
to an E
l
ectronegat
i
ve Atom
Alkyl halide:
C bonded to halogen (C
-
X)
Alkyl
halide:
C
bonded
to
halogen
(C
X)
Alcohol: C bonded O of a hydroxyl group (COH)
Ether: Two C’s bonded to the same O
(
COC
)
(
)
Amine: C bonded to N (CN)
Thiol: C bonded to SH group (CSH)
Sulfide: Two C’s bonded to same S (CSC)
Bonds are polar, with partial positive charge on C
(
+) and partial negative charge (
)on
(
+)
and
partial
negative
charge
(
)
on
electronegative atom

Groups with a Carbon–Oxygen Double
d( b l )
Bon
d
(
Car
b
ony
l
Groups
)
Bonds are polar with partial positive charge on
Bonds
are
polar
,
with
partial
positive
charge
on
C (+) and partial negative charge on O ()

**
**
**
**
**
**
**

**
**
**

**
**
**
**
**
**
**

Organic Structure Determination
Organic
Structure
Determination
S
p
ectrosco
py
= interaction of com
p
ounds with
ppy p
light (a form of energy)
E = h = hc/frequency, wavelength]
IR Spectroscopy = used to identify functional
groups within a compound

Absorption Spectroscopy
Or
g
anic com
p
ound ex
p
osed to electroma
g
netic
gpp g
radiation, can absorb energy of only certain
wavelengths (unit of energy)
Tit fth lth
T
ransm
it
s energy o
f
o
th
er wave
l
eng
th
s.
Changing wavelengths to determine which are
absorbed and which are transmitted produces an
absorbed
and
which
are
transmitted
produces
an
absorption spectrum
Energy absorbed is distributed internally in a distinct
ddibl
an
d
repro
d
uc
ibl
e way

Infrared (IR) Absorption
IR ener
gy
absor
p
tion corres
p
onds to s
p
ecific
gy p p p
vibrational and rotational modes, such as bending
and stretching of bonds
E i h t i ti f th t i th f ti l
E
nergy
i
s c
h
arac
t
er
i
s
ti
c o
f
th
e a
t
oms
i
n
th
e
f
unc
ti
ona
l
group and their bonding

Infrared (IR) Spectroscopy
IR ener
gy
in a s
p
ectrum is usuall
y
measured as
gy p y
wavenumber (cm
-1
), the inverse of wavelength and is
proportional to frequency and energy
Specific IR absorbed by organic molecule related to
Specific
IR
absorbed
by
organic
molecule
related
to
its bonding structure, principally its functional groups
Wavenumber = 1 / (cm)
_
= K √ f ( m
1
+ m
2
)
(m
m
)
stronger bonds = higher
heavier atoms
=
lower
_
_
_
(m
1
m
2
)
heavier
atoms
lower
Csp
3
-HCsp
3
-H
vs
Csp
2
-H
vs
vs
Csp
3
-Csp
3

Interpreting IR Spectra
Most functional
g
rou
p
s absorb at about the same ener
gy
gp gy
and intensity independent of the molecule they are in
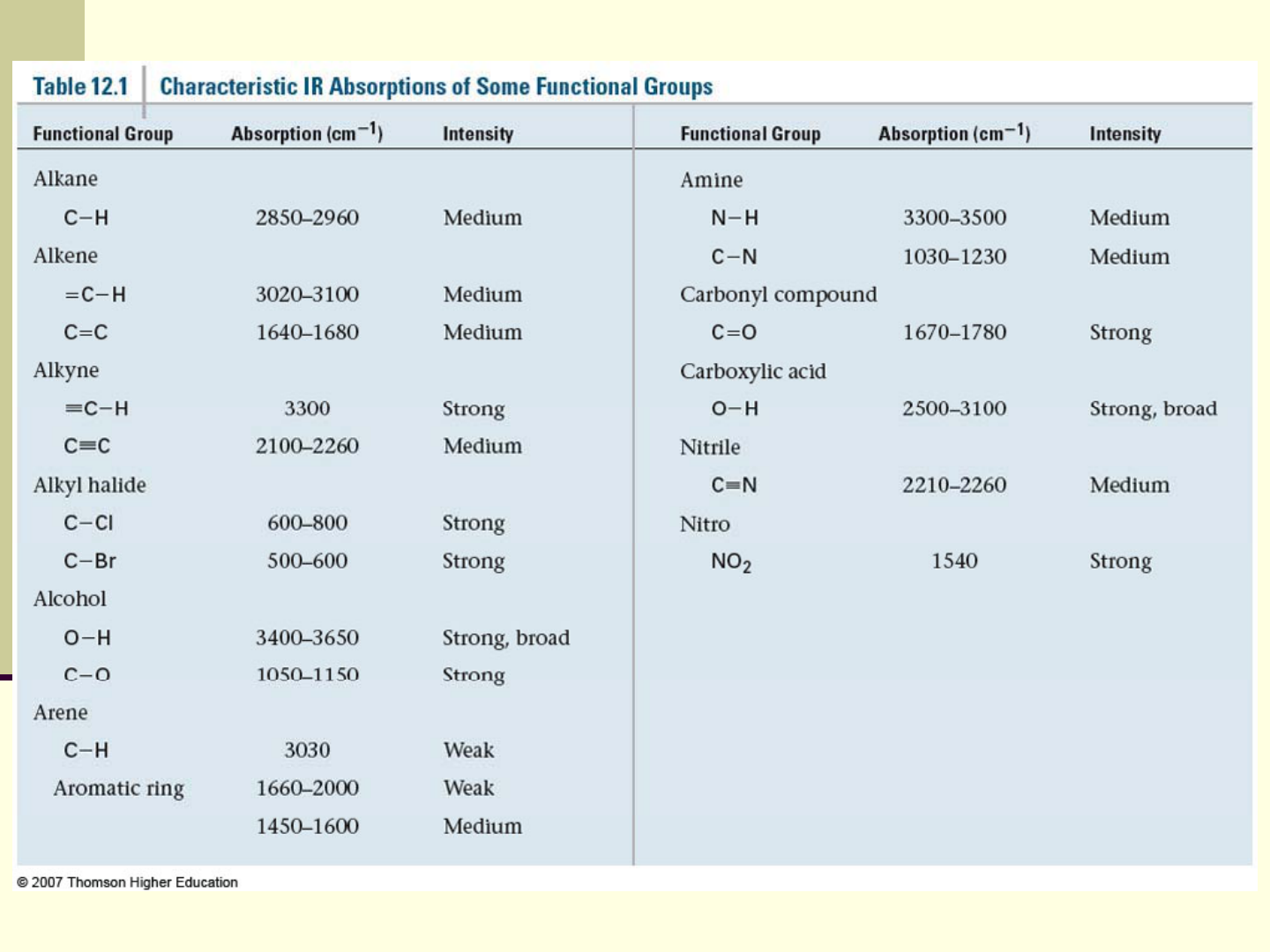
Characteristic higher energy IR absorptions in Table 12.1
can be used to confirm the existence of the presence of a
can
be
used
to
confirm
the
existence
of
the
presence
of
a
functional group in a molecule
IR spectrum has lower energy region characteristic of
molec le as a hole (“fingerprint” region belo 1500 cm
1
)
molec
u
le
as
a
w
hole
(“fingerprint”
region
belo
w
1500
cm
-
1
)
Look for "key" absorptions for functional groups, you
cannot assign all of the peaks (especially fingerprint
region that is unique to a compound)
Can only tell you what functional groups are in a
co
m
pou
n
d
(a
n
d
wh
at
f
u
n
ct
i
o
n
a
l
g
r
oups
a
r
e
n
ot
in
co pou d (a d at u ct o a g oups a e ot
compound). Cannot tell you how many or what exact
structure is.

Regions of the IR Absorption Spectrum
4000-2500 cm
-1
N-H,
C
HO
H (stretching)
2000-1500 cm
-1
double
bonds (stretching)
C
-
H
,
O
-
H
(stretching)
3300-3600 N-H, O-H
3000 C-H
2500
2000 cm
-
1
C
C and C
bonds
(stretching)
C=O 1680-1750
C=C 1640-1680 cm
-1
Below 1500
cm
-1
“
fingerprint
”
2500
-
2000
cm
-
1
C
C
and
C
N (stretching)
Below
1500
cm
fingerprint
region
** **
**
**
**
**
**
**
**
**
**
**
(two bands 1600 and 1500)
**
**
all values listed are for
bond stretching

IR of Hydrocarbons
Alkanes, Alkenes, Alk
y
nes
y
C-H, C-C, C=C, CC have characteristic peaks
based on bond strengths
absence helps rule out C=C or C
C
absence
helps
rule
out
C=C
or
C
C


IR of Aromatics
Weak C
H stretch at 3030 cm
1
Weak
C
–
H
stretch
at
3030
cm
1
Weak absorptions 1660 - 2000 cm
1
range
Medium
-
intensity absorptions 1450 to 1600 cm
1
Medium
intensity
absorptions
1450
to
1600
cm

IR of Alcohols and Amines
IR
of
Alcohols
and
Amines
O
–
H 3400 to 3650 cm
1
Usually broad and intense
N–H 3300 to 3500 cm
1
Sharper and less intense than an O–H
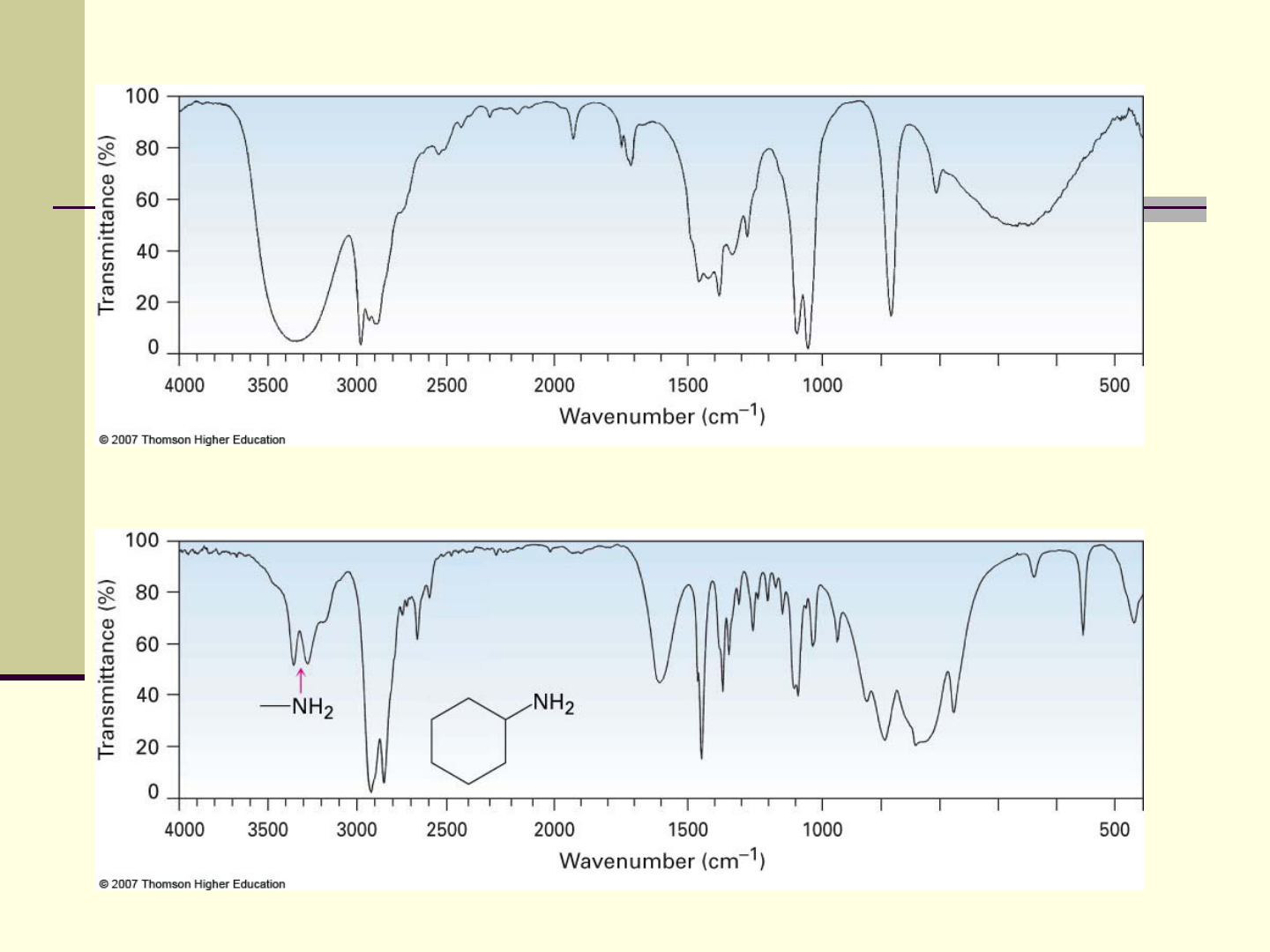
CH
3
CH
2
OH
3
2

IR of Carbonyl Compounds: Aldehydes
Stron
g,
shar
p
C=O
p
eak 1670 to 1780 cm
1
g, p p
Exact absorption characteristic of type of carbonyl
compound (ald, ket, ester, acid, amide, etc)
1730 cm
1
in saturated aldehydes
1705 cm
1
in aldehydes next to double bond or
aromatic ring
aromatic
ring

note Aldehyde C-H stretches at 2800-2700 cm
-1

IR of Ketones and Esters
1715
1i i
bdi d likt
1715
cm
1
i
n s
i
x-mem
b
ere
d
r
i
ng an
d
acyc
li
c
k
e
t
ones
1750 cm1 in 5-membered ring ketones
1690 cm1 in ketones next to a double bond or an aromatic ring
1735 cm1 in saturated esters
1715 cm1 in esters next to aromatic ring or a double bond

IR of Acids, Amides, Anhydrides, and
Acyl Halides
Carboxylic Acids:
Carboxylic
Acids:
O-H 2500-3300 cm-1 (very broad, strong)
C=O 1710-1760 cm1 (dimers lower, monomers higher )
Amides:
N-H 3300-3500 cm-1 (sharp, medium, varies with # of H's)
C=O 1690 cm
1 in saturated amides
Anh
yd
ri
des
:
yd des
C=O 1820 and 1760 cm-1 (two absorptions)
Acyl Halides:
Acyl
Halides:
C=O 1800cm-1

IR of Ketones and Acids
IR
of
Ketones
and
Acids

Match a structure from the list below to the IR spectrum
A. D.
HO
B. E.
O
O
O
OOH
C.
O
H
O
F.

Match a structure from the list below to the IR spectrum
A. D.
HO
Match
a
structure
from
the
list
below
to
the
IR
spectrum
B. E.
O
O
O
OOH
C.
O
H
O
F.

Match a structure from the list below to the IR spectrum
A. D.
HO
B. E.
O
O
O
OOH
C.
O
H
O
F.

Propose a structure with formula C
4
H
8
O that fits data

Propose a structure with formula C
4
H
8
O that fits data

How would you differentiate each pair of molecules
below using IR spectroscopy
below
using
IR
spectroscopy
(a)
and
(
b
)
a
n
d
OH NH
2
(
b
)
a
n
d
(c)
and
OH
NaOH
,
H
2
O
f
r
om
B
r
,
2





13
C NMR Spectroscopy Intro
Provides a method to count the number of different
(non-equivalent) carbons in a molecule
Will also give information about the chemical
environment around each carbon atom (ppm scale)
sp
3
C signal is at 0 to 9 sp
2
C: 110 to 220
C( O) at low field
160 to 220
C(
=
O)
at
low
field
,
160
to
220

Identify Equivalent Carbons
O
O
O
O
F
O
OH

13
C NMR Spectroscopy
How many signals would you expect to see in the
13
C NMR
spectrum of each of the following compounds?
O
OH

The Simplest FG: Alkanes
The
Simplest
FG:
Alkanes
Alkanes: Compounds with C-C single bonds and C-H
bonds only (no other functional groups)
Connecting carbons can lead to large or small molecules
The formula for an alkane with no rin
g
s in it must be
g
C
n
H
2n+2
where n is the number of carbon atoms
Alkanes are saturated with hydrogen (no more can be
added
They are also called aliphatic compounds
All C sp
3
hybridized with tetrahedral geometry (if no
charge)
charge)

Alkanes & Isomers
compounds with same molecular formula
but different arrangement of atoms
Alkanes
&
Isomers
CH
4
= methane
,
C
2
H
6
= ethane
,
C
3
H
8
=
p
ro
p
ane
but
different
arrangement
of
atoms
4
,
2
6
,
3
8
pp
The molecular formula of an alkane with more than
three carbons can give more than one structure
C
4
(butane) = butane and isobutane
C
5
(pentane) = pentane, 2-methylbutane, and 2,2-
dimeth
y
l
p
ro
p
ane
yp p
Alkanes with C’s connected to no more than 2 other
C’s are straight-chain or normal alkanes
A
lkanes with one or more C’s connected to 3 or 4 C’s
are branched-chain alkanes
isobutane
butane

Constitutional Isomers
isomers that differ by
atomic connectivity
Constitutional
Isomers
Isomers that differ in how their atoms are arran
g
ed in
atomic
connectivity
g
chains are called constitutional isomers
Compounds other than alkanes can be
constitutional isomers
of one another
constitutional
isomers
of
one
another
They must have the same molecular formula to be
isomers

Names of Normal Alkanes
Names
of
Normal
Alkanes
No. of Carbons Formula Name (C
n
H
2
n
+
2
)
1 Methane (Me) CH
4
2 Ethane (Et) C
2
H
6
3 Propane (Pr) C
3
H
8
4 Butane C
4
H
10
5
Pentane
C
5
H
12
5
Pentane
C
5
H
12
6 Hexane C
6
H
14
7 Heptane C
7
H
16
8 Octane C
8
H
18
9 Nonane C
9
H
20
10
D
C
H
10
D
ecane
C
10
H
22

Drawing Alkanes
Drawing
Alkanes
condensed drawings
skeletal drawing

Alkyl Groups
Alkyl
Groups
Alk
y
l
g
rou
p
–
remove one H from an alkane
(
a
p
art
yg p
(p
of a structure)
General abbreviation “R” (for Radical, an incomplete
species or the
“
rest
”
of the molecule)
species
or
the
rest
of
the
molecule)
Name: replace -ane ending of alkane with -yl ending
CH
3
is “methyl” (from methane)
CH
2
CH
3
is “ethyl” from ethane

Types of Alkyl groups
Types
of
Alkyl
groups
Classified b
y
the connection site
y
a carbon at the end of a chain (primary alkyl group)
a carbon with two other carbons attached to it
(dlkl )
(
secon
d
ary a
lk
y
l
group
)
a carbon with three other carbons attached to it
(tertiary alkyl group)
classify hydrogen in same fashion (1
o
H on 1
o
C)

Types of Alkyl groups
Types
of
Alkyl
groups
Some odd examples when non
-
carbon atoms
Some
odd
examples
when
non
carbon
atoms
are part of structure....
R-CH2-OH is primary C, primary H
need to fix HW answer key for this!
need
to
fix
HW
answer
key
for
this!
RCOH aldehyde is a primary carbon
RCOH
aldehyde
is
a
primary
carbon

Naming Alkanes
Naming
Alkanes
Compounds are given systematic names by a process that uses
Follows specific rules
Fi d t h d b h i
Fi
n
d
paren
t
h
y
d
rocar
b
on c
h
a
i
n
Carbons in that main chain are numbered in sequence
Substituents are identified numbered
Write compound name is single word
Name a complex substituents as though it were a compound
itsel
f
See specific examples in text

Naming Alkanes (IUPAC Rules)
Naming
Alkanes
(IUPAC
Rules)
1. Identif
y
the
p
arent
(
lon
g
est
)
chain
yp (g)
if choice, find one with the most branch points
2. Number atoms in this chain
number to give first branching group (substituent)
number
to
give
first
branching
group
(substituent)
lowest possible number
3. Name and number the substituents
if t o gro ps on same C gi e same n mber
if
t
w
o
gro
u
ps
on
same
C
,
gi
v
e
same
n
u
mber
if same group appears more than once, use di, tri..
replace -ane ending with -yl for substituents
4. Write name as a single word
use hyphens to separate numbers and letters
use commas to separate numbers
list subs alphabetically (don't consider di, tri.. sec-, tert-)
end name according to priority FG (ane for alkane)

Naming Alkanes
Naming
Alkanes
5. Name (complex substituents) by same rules
number substituent so that first atom connected
to main chain is position 1 (put in parenthesis)
6
Learn common names for branched substituents:
6
.
Learn
common
names
for
branched
substituents:
(when naming, can use common or IUPAC name)
iso part of name alphabetically
l(1
hl hl) b i
iso
part
of
name
alphabetically
,
sec- and tert- are not
a
l
so
(1
-met
h
y
l
et
h
y
l)
su
b
st
i
tuent

Examples
Examples

Examples
Examples
what if same numbers from both ends, go with alpha first lower numbe
r
given them some complex subs to name like
#
-
(2 3
-
dimethylbutyl)
#
(2
,
3
dimethylbutyl)
etc

Physical Properties
Physical
Properties
Boiling points and melting points increase as
Boiling
points
and
melting
points
increase
as
size of alkane increases
Dis
p
ersion forces increase as molecule size
p
increases, resulting in higher melting and
boiling points
